Recurrent mossy fibers establish aberrant kainate receptor-operated synapses on granule cells from epileptic rats
- PMID: 16148230
- PMCID: PMC6725550
- DOI: 10.1523/JNEUROSCI.1469-05.2005
Recurrent mossy fibers establish aberrant kainate receptor-operated synapses on granule cells from epileptic rats
Abstract
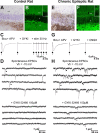
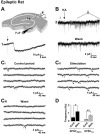
Glutamatergic mossy fibers of the hippocampus sprout in temporal lobe epilepsy and establish aberrant synapses on granule cells from which they originate. There is currently no evidence for the activation of kainate receptors (KARs) at recurrent mossy fiber synapses in epileptic animals, despite their important role at control mossy fiber synapses. We report that KARs are involved in ongoing glutamatergic transmission in granule cells from chronic epileptic but not control animals. KARs provide a substantial component of glutamatergic activity, because they support half of the non-NMDA receptor-mediated excitatory drive in these cells. KAR-mediated EPSC(KA)s are selectively generated by recurrent mossy fiber inputs and have a slower kinetics than EPSC(AMPA). Therefore, in addition to axonal rewiring, sprouting of mossy fibers induces a shift in the nature of glutamatergic transmission in granule cells that may contribute to the physiopathology of the dentate gyrus in epileptic animals.
Figures






References
-
- Ben-Ari Y (1985) Limbic seizure and brain-damage produced by kainic acid–mechanisms and relevance to human temporal-lobe epilepsy. Neuroscience 14: 375–403. - PubMed
-
- Ben-Ari Y, Cossart R (2000) Kainate, a double agent that generates seizures: two decades of progress. Trends Neurosci 23: 580–587. - PubMed
-
- Ben-Ari Y, Represa A (1990) Brief seizure episodes induce long-term potentiation and mossy fiber sprouting in the hippocampus. Trends Neurosci 13: 312–318. - PubMed
-
- Berry MS, Pentreath VW (1976) Criteria for distinguishing between monosynaptic and polysynaptic transmission. Brain Res 105: 1–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
