Ionic mechanisms underlying autonomous action potential generation in the somata and dendrites of GABAergic substantia nigra pars reticulata neurons in vitro
- PMID: 16148235
- PMCID: PMC6725542
- DOI: 10.1523/JNEUROSCI.1475-05.2005
Ionic mechanisms underlying autonomous action potential generation in the somata and dendrites of GABAergic substantia nigra pars reticulata neurons in vitro
Abstract
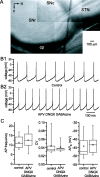
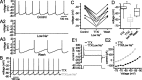
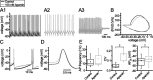
Through their repetitive discharge, GABAergic neurons of the substantia nigra pars reticulata (SNr) tonically inhibit the target nuclei of the basal ganglia and the dopamine neurons of the midbrain. As the repetitive firing of SNr neurons persists in vitro, perforated, whole-cell and cell-attached patch-clamp recordings were made from rat brain slices to determine the mechanisms underlying this activity. The spontaneous activity of SNr neurons was not perturbed by the blockade of fast synaptic transmission, demonstrating that it was autonomous in nature. A subthreshold, slowly inactivating, voltage-dependent, tetrodotoxin (TTX)-sensitive Na+ current and a TTX-insensitive inward current that was mediated in part by Na+ were responsible for depolarization to action potential (AP) threshold. An apamin-sensitive spike afterhyperpolarization mediated by small-conductance Ca2+-dependent K+ (SK) channels was critical for the precision of autonomous activity. SK channels were activated, in part, by Ca(2+) flowing throughomega-conotoxin GVIA-sensitive, class 2.2 voltage-dependent Ca2+ channels. Although Cs+/ZD7288 (4-ethylphenylamino-1,2-dimethyl-6-methylaminopyrimidinium chloride)-sensitive hyperpolarization-activated currents were also observed in SNr neurons, they were activated at voltages that were in general more hyperpolarized than those associated with autonomous activity. Simultaneous somatic and dendritic recordings revealed that autonomously generated APs were observed first at the soma before propagating into dendrites up to 120 microm from the somatic recording site. Backpropagation of autonomously generated APs was reliable with no observable incidence of failure. Together, these data suggest that the resting inhibitory output of the basal ganglia relies, in large part, on the intrinsic firing properties of the neurons that convey this signal.
Figures




References
-
- Barry PH (1994) JPCalc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. J Neurosci Methods 51: 107–116. - PubMed
-
- Bentivoglio M, van der Kooy D, Kuypers HG (1979) The organization of the efferent projections of the substantia nigra in the rat. A retrograde fluorescent double labeling study. Brain Res 174: 1–17. - PubMed
-
- Beurrier C, Bioulac B, Hammond C (2000) Slowly inactivating sodium current (INaP) underlies single-spike activity in rat subthalamic neurons. J Neurophysiol 83: 1951–1957. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
