Posttranslational protein modification in Archaea
- PMID: 16148304
- PMCID: PMC1197805
- DOI: 10.1128/MMBR.69.3.393-425.2005
Posttranslational protein modification in Archaea
Erratum in
- Microbiol Mol Biol Rev. 2005 Dec;69(4):696
Abstract
One of the first hurdles to be negotiated in the postgenomic era involves the description of the entire protein content of the cell, the proteome. Such efforts are presently complicated by the various posttranslational modifications that proteins can experience, including glycosylation, lipid attachment, phosphorylation, methylation, disulfide bond formation, and proteolytic cleavage. Whereas these and other posttranslational protein modifications have been well characterized in Eucarya and Bacteria, posttranslational modification in Archaea has received far less attention. Although archaeal proteins can undergo posttranslational modifications reminiscent of what their eucaryal and bacterial counterparts experience, examination of archaeal posttranslational modification often reveals aspects not previously observed in the other two domains of life. In some cases, posttranslational modification allows a protein to survive the extreme conditions often encountered by Archaea. The various posttranslational modifications experienced by archaeal proteins, the molecular steps leading to these modifications, and the role played by posttranslational modification in Archaea form the focus of this review.
Figures



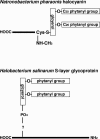


Similar articles
-
Modifying Post-Translational Modifications: A Strategy Used by Archaea for Adapting to Changing Environments?: Manipulating the Extent, Position, or Content of Post-Translational Modifications May Help Archaea Adapt to Environmental Change.Bioessays. 2020 Mar;42(3):e1900207. doi: 10.1002/bies.201900207. Epub 2020 Jan 29. Bioessays. 2020. PMID: 31994760
-
N-glycosylation in Archaea-New roles for an ancient posttranslational modification.Mol Microbiol. 2020 Nov;114(5):735-741. doi: 10.1111/mmi.14569. Epub 2020 Jul 26. Mol Microbiol. 2020. PMID: 32633872 Review.
-
Sweet to the extreme: protein glycosylation in Archaea.Mol Microbiol. 2008 Jun;68(5):1079-84. doi: 10.1111/j.1365-2958.2008.06224.x. Mol Microbiol. 2008. PMID: 18476920 Review.
-
Sweet New Roles for Protein Glycosylation in Prokaryotes.Trends Microbiol. 2017 Aug;25(8):662-672. doi: 10.1016/j.tim.2017.03.001. Epub 2017 Mar 21. Trends Microbiol. 2017. PMID: 28341406 Review.
-
Protein glycosylation in Archaea: sweet and extreme.Glycobiology. 2010 Sep;20(9):1065-76. doi: 10.1093/glycob/cwq055. Epub 2010 Apr 5. Glycobiology. 2010. PMID: 20371512 Review.
Cited by
-
Protein phosphorylation and its role in archaeal signal transduction.FEMS Microbiol Rev. 2016 Sep;40(5):625-47. doi: 10.1093/femsre/fuw020. Epub 2016 Jul 29. FEMS Microbiol Rev. 2016. PMID: 27476079 Free PMC article. Review.
-
Structure-guided identification of a new catalytic motif of oligosaccharyltransferase.EMBO J. 2008 Jan 9;27(1):234-43. doi: 10.1038/sj.emboj.7601940. Epub 2007 Nov 29. EMBO J. 2008. PMID: 18046457 Free PMC article.
-
Towards glycoengineering in archaea: replacement of Haloferax volcanii AglD with homologous glycosyltransferases from other halophilic archaea.Appl Environ Microbiol. 2010 Sep;76(17):5684-92. doi: 10.1128/AEM.00681-10. Epub 2010 Jul 2. Appl Environ Microbiol. 2010. PMID: 20601508 Free PMC article.
-
Identification of the archaeal alg7 gene homolog (encoding N-acetylglucosamine-1-phosphate transferase) of the N-linked glycosylation system by cross-domain complementation in Saccharomyces cerevisiae.J Bacteriol. 2008 Mar;190(6):2217-20. doi: 10.1128/JB.01778-07. Epub 2008 Jan 4. J Bacteriol. 2008. PMID: 18178736 Free PMC article.
-
Di-myo-inositol phosphate and novel UDP-sugars accumulate in the extreme hyperthermophile Pyrolobus fumarii.Extremophiles. 2008 May;12(3):383-9. doi: 10.1007/s00792-008-0143-0. Epub 2008 Feb 20. Extremophiles. 2008. PMID: 18286223
References
-
- Akca, E., H. Claus, N. Schultz, G. Karbach, B. Schlott, T. Debaerdemaeker, J. P. Declercq, and H. Konig. 2002. Genes and derived amino acid sequences of S-layer proteins from mesophilic, thermophilic, and extremely thermophilic methanococci. Extremophiles 6:351-358. - PubMed
-
- Albers, S. V., and A. J. M. Driessen. 2002. Signal peptides of secreted proteins of the archaeon Sulfolobus solfataricus: a genomic survey. Arch. Microbiol. 177:209-216. - PubMed
-
- Albers, S. V., S. M. Koning, W. N. Konings, and A. J. Driessen. 2004. Insights into ABC transport in archaea. J. Bioenerg. Biomembr. 36:5-15. - PubMed
-
- Albers, S. V., W. N. Konings, and A. J. Driessen. 1999. A unique short signal sequence in membrane-anchored proteins of Archaea. Mol. Microbiol. 31:1595-1596. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

