Wound-healing defect of CD18(-/-) mice due to a decrease in TGF-beta1 and myofibroblast differentiation
- PMID: 16148944
- PMCID: PMC1276170
- DOI: 10.1038/sj.emboj.7600809
Wound-healing defect of CD18(-/-) mice due to a decrease in TGF-beta1 and myofibroblast differentiation
Abstract
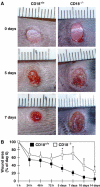
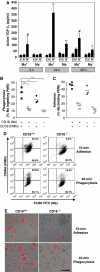
We studied the mechanisms underlying the severely impaired wound healing associated with human leukocyte-adhesion deficiency syndrome-1 (LAD1) using a murine disease model. In CD18(-/-) mice, healing of full-thickness wounds was severely delayed during granulation-tissue contraction, a phase where myofibroblasts play a major role. Interestingly, expression levels of myofibroblast markers alpha-smooth muscle actin and ED-A fibronectin were substantially reduced in wounds of CD18(-/-) mice, suggesting an impaired myofibroblast differentiation. TGF-beta signalling was clearly involved since TGF-beta1 and TGF-beta receptor type-II protein levels were decreased, while TGF-beta(1) injections into wound margins fully re-established wound closure. Since, in CD18(-/-) mice, defective migration leads to a severe reduction of neutrophils in wounds, infiltrating macrophages might not phagocytose apoptotic CD18(-/-) neutrophils. Macrophages would thus be lacking their main stimulus to secrete TGF-beta1. Indeed, in neutrophil-macrophage cocultures, lack of CD18 on either cell type leads to dramatically reduced TGF-beta1 release by macrophages due to defective adhesion to, and subsequent impaired phagocytic clearance of, neutrophils. Our data demonstrates that the paracrine secretion of growth factors is essential for cellular differentiation in wound healing.
Figures





References
-
- Anderson DC, Schmalstieg FC, Arnaout MA, Kohl S, Tosi MF, Dana N, Buffone GJ, Hughes BJ, Brinkley BR, Dickey WD, Abramson JS, Springer T, Boxer LA, Hollers JM, Smith CW (1984) Abnormalities of polymorphonuclear leukocyte function associated with a heritable deficiency of high molecular weight surface glycoproteins (GP138): common relationship to diminished cell adherence. J Clin Invest 74: 536–551 - PMC - PubMed
-
- Anderson DC, Smith CW (2001) Leukocyte Adhesion Deficiencies. New York, NY: McGraw-Hill
-
- Angele MK, Knoferl MW, Ayala A, Albina JE, Cioffi WG, Bland KI, Chaudry IH (1999) Trauma-hemorrhage delays wound healing potentially by increasing pro-inflammatory cytokines at the wound site. Surgery 126: 279–285 - PubMed
-
- Arnaout MA (1990) Structure and function of the leukocyte adhesion molecules CD11/CD18. Blood 75: 1037–1050 - PubMed
-
- Arora PD, McCulloch CA (1994) Dependence of collagen remodelling on alpha-smooth muscle actin expression by fibroblasts. J Cell Physiol 159: 161–175 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

