Ubiquitylation and cell signaling
- PMID: 16148945
- PMCID: PMC1276169
- DOI: 10.1038/sj.emboj.7600808
Ubiquitylation and cell signaling
Abstract
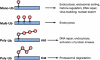
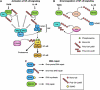
Ubiquitylation is an emerging mechanism implicated in a variety of nonproteolytic cellular functions. The attachment of a single ubiquitin (Ub) or poly-Ub (lysine 63) chains to proteins control gene transcription, DNA repair and replication, intracellular trafficking and virus budding. In these processes, protein ubiquitylation exhibits inducibility, reversibility and recognition by specialized domains, features similar to protein phosphorylation, which enable Ub to act as a signaling device. Here, we highlight several recent examples on how Ub regulates signaling and how signaling regulates ubiquitylation during physiological and pathological cellular processes.
Figures
References
-
- Adams J, Kauffman M (2004) Development of the proteasome inhibitor Velcade (Bortezomib). Cancer Invest 22: 304–311 - PubMed
-
- Blume-Jensen P, Hunter T (2001) Oncogenic kinase signalling. Nature 411: 355–365 - PubMed
-
- Brummelkamp TR, Nijman SM, Dirac AM, Bernards R (2003) Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-kappaB. Nature 424: 797–801 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

