Labeling fibroblasts with biotin-BSA-GdDTPA-FAM for tracking of tumor-associated stroma by fluorescence and MR imaging
- PMID: 16149062
- PMCID: PMC1382177
- DOI: 10.1002/mrm.20628
Labeling fibroblasts with biotin-BSA-GdDTPA-FAM for tracking of tumor-associated stroma by fluorescence and MR imaging
Abstract
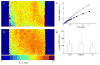
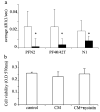
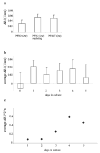
Fibroblasts at the tumor-host interface can differentiate into myofibroblasts and pericytes, and contribute to the guidance and stabilization of endothelial sprouts. After intravenous administration of biotin-BSA-GdDTPA-FAM in mice with subcutaneous MLS human ovarian carcinoma tumors, the distribution of the macromolecular MRI/optical contrast material was confined to blood vessels in normal tissues, while it co-registered with alphaSMA-positive stroma tracks within the tumor. These alphaSMA-positive tumor-associated myofibroblasts and pericytes showed uptake of the contrast material into intracellular granules. We evaluated the use of this contrast material for in vitro labeling of tumor fibroblasts as an approach for tracking their involvement in angiogenesis. Fluorescence microscopy demonstrated internalization of the contrast material, and MRI revealed a significant increase in the R(1) relaxation rate of labeled fibroblasts. R(1) not only remained elevated for 2 weeks in culture, it also increased with cell proliferation, indicating prolonged retention of the contrast material and subsequent intracellular processing and redistribution of the material, and thereby enhancing MR contrast. Moreover, cells that were labeled ex vivo with MR contrast material and co-inoculated with tumor cells in mice were detected in vivo by MRI. Uptake of the contrast material was suppressed by nystatin, suggesting internalization by caveolae-mediated endocytosis. This study shows that labeling of fibroblasts with biotin-BSA-GdDTPA-FAM is feasible and would allow noninvasive in vivo tracking of fibroblasts during tumor angiogenesis and vessel maturation.
Copyright 2005 Wiley-Liss, Inc.
Figures



Similar articles
-
Biotinylated bovine serum albumin linked to gadolinium diethylenetriaminepentaacetic acid.2008 Dec 9 [updated 2008 Dec 26]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2008 Dec 9 [updated 2008 Dec 26]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641367 Free Books & Documents. Review.
-
Modulation of the pharmacokinetics of macromolecular contrast material by avidin chase: MRI, optical, and inductively coupled plasma mass spectrometry tracking of triply labeled albumin.Magn Reson Med. 2003 Nov;50(5):904-14. doi: 10.1002/mrm.10638. Magn Reson Med. 2003. PMID: 14587000
-
In vivo imaging of the systemic recruitment of fibroblasts to the angiogenic rim of ovarian carcinoma tumors.Cancer Res. 2007 Oct 1;67(19):9180-9. doi: 10.1158/0008-5472.CAN-07-0684. Cancer Res. 2007. PMID: 17909023 Free PMC article.
-
AG-013736, a novel inhibitor of VEGF receptor tyrosine kinases, inhibits breast cancer growth and decreases vascular permeability as detected by dynamic contrast-enhanced magnetic resonance imaging.Magn Reson Imaging. 2007 Apr;25(3):319-27. doi: 10.1016/j.mri.2006.09.041. Epub 2007 Feb 5. Magn Reson Imaging. 2007. PMID: 17371720
-
MR mammography with pharmacokinetic mapping for monitoring of breast cancer treatment during neoadjuvant therapy.Magn Reson Imaging Clin N Am. 1994 Nov;2(4):633-58. Magn Reson Imaging Clin N Am. 1994. PMID: 7489314 Review.
Cited by
-
Mesenchymal stem cell-derived microvesicles protect against acute tubular injury.J Am Soc Nephrol. 2009 May;20(5):1053-67. doi: 10.1681/ASN.2008070798. Epub 2009 Apr 23. J Am Soc Nephrol. 2009. PMID: 19389847 Free PMC article.
-
Functional phenotyping of the maternal albumin turnover in the mouse placenta by dynamic contrast-enhanced MRI.Mol Imaging Biol. 2011 Jun;13(3):481-492. doi: 10.1007/s11307-010-0390-1. Mol Imaging Biol. 2011. PMID: 20686857 Free PMC article.
-
Characterization of the Tumor Microenvironment and Tumor-Stroma Interaction by Non-invasive Preclinical Imaging.Front Oncol. 2017 Jan 31;7:3. doi: 10.3389/fonc.2017.00003. eCollection 2017. Front Oncol. 2017. PMID: 28197395 Free PMC article. Review.
-
Harnessing competing endocytic pathways for overcoming the tumor-blood barrier: magnetic resonance imaging and near-infrared imaging of bifunctional contrast media.Cancer Res. 2009 Jul 1;69(13):5610-7. doi: 10.1158/0008-5472.CAN-08-4967. Epub 2009 Jun 9. Cancer Res. 2009. PMID: 19509228 Free PMC article.
-
MR tracking of transplanted cells with "positive contrast" using manganese oxide nanoparticles.Magn Reson Med. 2008 Jul;60(1):1-7. doi: 10.1002/mrm.21622. Magn Reson Med. 2008. PMID: 18581402 Free PMC article.
References
-
- De Wever O, Mareel M. Role of tissue stroma in cancer cell invasion. J Pathol. 2003;200(4):429–447. - PubMed
-
- Elenbaas B, Weinberg RA. Heterotypic signaling between epithelial tumor cells and fibroblasts in carcinoma formation. Exp Cell Res. 2001;264(1):169–184. - PubMed
-
- Kunz-Schughart LA, Knuechel R. Tumor-associated fibroblasts (part II): Functional impact on tumor tissue. Histol Histopathol. 2002;17(2):623–637. - PubMed
-
- Kunz-Schughart LA, Knuechel R. Tumor-associated fibroblasts (part I): Active stromal participants in tumor development and progression? Histol Histopathol. 2002;17(2):599–621. - PubMed
-
- Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315(26):1650–1659. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

