Myocardial contractility in the echo lab: molecular, cellular and pathophysiological basis
- PMID: 16150150
- PMCID: PMC1242240
- DOI: 10.1186/1476-7120-3-27
Myocardial contractility in the echo lab: molecular, cellular and pathophysiological basis
Abstract
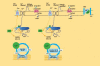
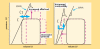
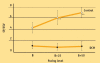
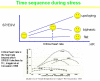
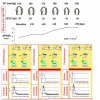
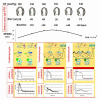
In the standard accepted concept, contractility is the intrinsic ability of heart muscle to generate force and to shorten, independently of changes in the preload or afterload with fixed heart rates. At molecular level the crux of the contractile process lies in the changing concentrations of Ca2+ ions in the myocardial cytosol. Ca2+ ions enter through the calcium channel that opens in response to the wave of depolarization that travels along the sarcolemma. These Ca2+ ions "trigger" the release of more calcium from the sarcoplasmic reticulum (SR) and thereby initiate a contraction-relaxation cycle. In the past, several attempts were made to transfer the pure physiological concept of contractility, expressed in the isolated myocardial fiber by the maximal velocity of contraction of unloaded muscle fiber (Vmax), to the in vivo beating heart. Suga and Sagawa achieved this aim by measuring pressure/volume loops in the intact heart: during a positive inotropic intervention, the pressure volume loop reflects a smaller end-systolic volume and a higher end-systolic pressure, so that the slope of the pressure volume relationship moves upward and to the left. The pressure volume relationship is the most reliable index for assessing myocardial contractility in the intact circulation and is almost insensitive to changes in preload and after load. This is widely used in animal studies and occasionally clinically. The limit of the pressure volume relationship is that it fails to take into account the frequency-dependent regulation of contractility: the frequency-dependent control of transmembrane Ca2+ entry via voltage-gated Ca2+ channels provides cardiac cells with a highly sophisticated short-term system for the regulation of intracellular Ca2+ homeostasis. An increased stimulation rate increases the force of contraction: the explanation is repetitive Ca2+ entry with each depolarization and, hence, an accumulation of cytosolic calcium. As the heart fails, there is a change in the gene expression from the normal adult pattern to that of fetal life with an inversion of the normal positive slope of the force-frequency relation: systolic calcium release and diastolic calcium reuptake process is lowered at the basal state and, instead of accelerating for increasing heart rates, slows down. Since the force-frequency relation uncovers initial alteration of contractility, as an intermediate step between normal and abnormal contractility at rest, a practical index to measure it is mandatory. Measuring end-systolic elastance for increasing heart rates is impractical: increasing heart rates with atrial pacing has to be adjunct to the left ventricular conductance catheter, to the left ventricular pressure catheter, to the vena cava balloon, and to afterload changes. Furthermore, a noninvasive index is needed. Noninvasive measurement of the pressure/volume ratio for increasing heart rates during stress in the echo lab could be the practical answer to this new clinical demand in the current years of a dramatic increase in the number of heart failure patients.
Figures










References
-
- Franciolini F, Petris A. Evolution of ionic Channels of biological membranes. Mol Biol Evol. 1989;6:503–513. - PubMed
-
- Bowditch HP. Über die Eigenthüm-lichkeiten der Reizbarkeit, welche die Muskelfasern des Herzens zeigen. Ber Sächs Akad Wiss. 1871;23:652–689.
-
- Piot C, Lemaire S, Albat B, Seguin J, Nargeot J, Richard S. High frequency-induced upregulation of human cardiac calcium currents. Circulation. 1996;93:120–128. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

