Mutation of key residues of RPE65 abolishes its enzymatic role as isomerohydrolase in the visual cycle
- PMID: 16150724
- PMCID: PMC1224626
- DOI: 10.1073/pnas.0504167102
Mutation of key residues of RPE65 abolishes its enzymatic role as isomerohydrolase in the visual cycle
Abstract
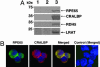
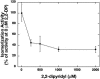
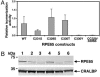
RPE65 is essential for isomerization of vitamin A to the visual chromophore. Mutations in RPE65 cause early-onset blindness, and Rpe65-deficient mice lack 11-cis-retinal but overaccumulate alltrans-retinyl esters in the retinal pigment epithelium (RPE). RPE65 is proposed to be a substrate chaperone but may have an enzymatic role because it is closely related to carotenoid oxygenases. We hypothesize that, by analogy with other carotenoid oxygenases, the predicted iron-coordinating residues of RPE65 are essential for retinoid isomerization. To clarify RPE65's role in isomerization, we reconstituted a robust minimal visual cycle in 293-F cells. Only cells transfected with RPE65 constructs produced 11-cis-retinoids, but coexpression with lecithin:retinol acyltransferase was needed for high-level production. Accumulation was significant, amounting to >2 nmol of 11-cis-retinol per culture. Transfection with constructs harboring mutations in residues of RPE65 homologous to those required for interlinked enzymatic activity and iron coordination in related enzymes abolish this isomerization. Iron chelation also abolished isomerization activity. Mutating cysteines implicated in palmitoylation of RPE65 had generally little effect on isomerization activity. Mutations associated with Leber congenital amaurosis/early-onset blindness cause partial to total loss of isomerization activity in direct relation to their clinical effects. These findings establish a catalytic role, in conjunction with lecithin:retinol acyltransferase, for RPE65 in synthesis of 11-cis-retinol, and its identity as the isomerohydrolase.
Figures



References
-
- Ruiz, A., Winston, A., Lim, Y. H., Gilbert, B. A., Rando, R. R. & Bok, D. (1999) J. Biol. Chem. 274, 3834-3841. - PubMed
-
- Saari, J. C., Bredberg, D. L. & Noy, N. (1994) Biochemistry 33, 3106-3112. - PubMed
-
- Deigner, P. S., Law, W. C., Canada, F. J. & Rando, R. R. (1989) Science 244, 968-971. - PubMed
-
- Moiseyev, G., Crouch, R. K., Goletz, P., Oatis, J., Jr., Redmond, T. M. & Ma, J. X. (2003) Biochemistry 42, 2229-2238. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

