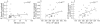Assessment of the clinical significance of gelatinase activity in patients with juvenile idiopathic arthritis using quantitative protein substrate zymography
- PMID: 16150790
- PMCID: PMC1798108
- DOI: 10.1136/ard.2005.039032
Assessment of the clinical significance of gelatinase activity in patients with juvenile idiopathic arthritis using quantitative protein substrate zymography
Abstract
Objective: To measure gelatinase activities in paired synovial fluid (SF) and serum of patients with juvenile idiopathic arthritis (JIA), and to assess how these activities relate to clinical and laboratory measures of disease activity.
Methods: A quantitative protein substrate zymography method was adapted and validated for use with serum and SF. Bands of activity were measured by densitometry and correlated with standard laboratory indicators of inflammation: erythrocyte sedimentation rate and platelet count.
Results: Gelatinase activity was found consistently in patients with JIA, with reproducible, quantified bands of activity corresponding to pro-matrix metalloproteinase-9 (pro-MMP-9), including the neutrophil associated lipocalin complex, and pro- and active forms of MMP-2. Both active MMP-2 and pro-MMP-9 were higher in JIA serum than in controls, though no differences were seen between patients grouped according to age, disease duration, or JIA subtype. However, SF MMP-9 correlated significantly with the laboratory indicators of inflammation, as did the relative level of active MMP-2.
Conclusions: Both MMP-2 and MMP-9 gelatinolytic activities are raised during active JIA and associated with inflammatory activity regardless of age and disease duration, supporting a role for MMPs in the breakdown of joint components from early in disease. These MMPs may be specific markers of active joint destruction linked to inflammatory JIA, MMP-9 as a product of infiltrating cells, and the activation of MMP-2 produced within the joint.
References
-
- Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 200392827–839. - PubMed
-
- Springman E B, Angleton E L, Birkedal‐Hansen H, Van Wart H E. Multiple modes of activation of latent human fibroblast collagenase: evidence for the role of a Cys73 active‐site zinc complex in latency and a “cysteine switch” mechanism for activation. Proc Natl Acad Sci USA 199087364–368. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous