Effect of temperature on the nanomechanics of lipid bilayers studied by force spectroscopy
- PMID: 16150966
- PMCID: PMC1366991
- DOI: 10.1529/biophysj.105.065581
Effect of temperature on the nanomechanics of lipid bilayers studied by force spectroscopy
Abstract
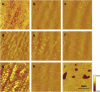
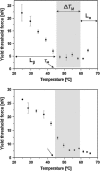
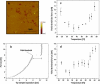
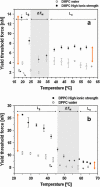
The effect of temperature on the nanomechanical response of supported lipid bilayers has been studied by force spectroscopy with atomic force microscopy. We have experimentally proved that the force needed to puncture the lipid bilayer (Fy) is temperature dependent. The quantitative measurement of the evolution of Fy with temperature has been related to the structural changes that the surface undergoes as observed through atomic force microscopy images. These studies were carried out with three different phosphatidylcholine bilayers with different main phase transition temperature (TM), namely, 1,2-dimyristoyl-sn-glycero-3-phosphocholine, 1,2-dipalmitoyl-sn-glycero-3-phosphocholine, and 2-dilauroyl-sn-glycero-3-phosphocholine. The solid-like phase shows a much higher Fy than the liquid-like phase, which also exhibits a jump in the force curve. Within the solid-like phase, Fy decreases as temperature is increased and suddenly drops as it approaches TM. Interestingly, a "well" in the Fy versus temperature plot occurs around TM, thus proving an "anomalous mechanical softening" around TM. Such mechanical softening has been predicted by experimental techniques and also by molecular dynamics simulations and interpreted in terms of water ordering around the phospholipid headgroups. Ion binding has been demonstrated to increase Fy, and its influence on both solid and liquid phases has also been discussed.
Figures




References
-
- Nagle, J. F., H. I. Petrache, N. Gouliaev, S. Tristram-Nagle, Y. F. Liu, R. M. Suter, and K. Gawrisch. 1998. Multiple mechanisms for critical behavior in the biologically relevant phase of lecithin bilayers. Phys. Rev. 58:7769–7776.
-
- Makino, K., T. Yamada, M. Kimura, T. Oka, H. Ohshima, and T. Kondo. 1991. Temperature- and ionic strength-induced conformational changes in the lipid head group region of liposomes as suggested by zeta potential data. Biophys. Chem. 41:175–183. - PubMed
-
- Wu, S. H. W., and H. M. McConnel. 1973. Lateral phase separations and perpendicular transport in membranes. Biochem. Biophys. Res. Commun. 55:484–491. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

