Molecular identification and reconstitution of depolarization-induced exocytosis monitored by membrane capacitance
- PMID: 16150968
- PMCID: PMC1367000
- DOI: 10.1529/biophysj.105.064642
Molecular identification and reconstitution of depolarization-induced exocytosis monitored by membrane capacitance
Abstract
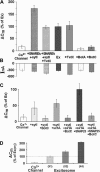
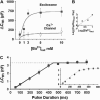
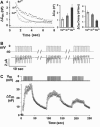
Regulated exocytosis of neurotransmitters at synapses is fast and tightly regulated. It is unclear which proteins constitute the "minimal molecular machinery" for this process. Here, we show that a novel technique of capacitance monitoring combined with heterologous protein expression can be used to reconstitute exocytosis that is fast (<0.5 s) and triggered directly by membrane depolarization in Xenopus oocytes. Testing synaptic proteins, voltage-gated Ca2+ channels, and using botulinum and tetanus neurotoxins established that the expression of a Ca2+ channel together with syntaxin 1A, SNAP-25, and synaptotagmin was sufficient and necessary for the reconstitution of depolarization-induced exocytosis. Similar to synaptic exocytosis, the reconstituted release was sensitive to neurotoxins, modulated by divalent cations (Ca2+, Ba2+, and Sr2+) or channel (Lc-, N-type), and depended nonlinearly on divalent cation concentration. Because of its improved speed, native trigger, and great experimental versatility, this reconstitution assay provides a novel, promising tool to study synaptic exocytosis.
Figures


Similar articles
-
Reconstitution of depolarization and Ca2+-evoked secretion in Xenopus oocytes monitored by membrane capacitance.Methods Mol Biol. 2008;440:269-82. doi: 10.1007/978-1-59745-178-9_21. Methods Mol Biol. 2008. PMID: 18369953
-
R-type voltage-gated Ca(2+) channel interacts with synaptic proteins and recruits synaptotagmin to the plasma membrane of Xenopus oocytes.Neuroscience. 2004;128(4):831-41. doi: 10.1016/j.neuroscience.2004.07.027. Neuroscience. 2004. PMID: 15464290
-
Ion interaction at the pore of Lc-type Ca2+ channel is sufficient to mediate depolarization-induced exocytosis.J Neurochem. 2006 Apr;97(1):116-27. doi: 10.1111/j.1471-4159.2006.03709.x. Epub 2006 Mar 3. J Neurochem. 2006. PMID: 16515555
-
Functional and physical coupling of voltage-sensitive calcium channels with exocytotic proteins: ramifications for the secretion mechanism.J Neurochem. 2001 May;77(4):972-85. doi: 10.1046/j.1471-4159.2001.00347.x. J Neurochem. 2001. PMID: 11359862 Review.
-
Interactions between proteins implicated in exocytosis and voltage-gated calcium channels.Philos Trans R Soc Lond B Biol Sci. 1999 Feb 28;354(1381):289-97. doi: 10.1098/rstb.1999.0380. Philos Trans R Soc Lond B Biol Sci. 1999. PMID: 10212477 Free PMC article. Review.
Cited by
-
Non-ionotropic voltage-gated calcium channel signaling.Channels (Austin). 2024 Dec;18(1):2341077. doi: 10.1080/19336950.2024.2341077. Epub 2024 Apr 11. Channels (Austin). 2024. PMID: 38601983 Free PMC article. Review.
-
The L-type Voltage-Gated Calcium Channel co-localizes with Syntaxin 1A in nano-clusters at the plasma membrane.Sci Rep. 2017 Sep 12;7(1):11350. doi: 10.1038/s41598-017-10588-4. Sci Rep. 2017. PMID: 28900128 Free PMC article.
-
Structure of human synaptotagmin 1 C2AB in the absence of Ca2+ reveals a novel domain association.Biochemistry. 2007 Nov 13;46(45):13041-8. doi: 10.1021/bi701651k. Epub 2007 Oct 23. Biochemistry. 2007. PMID: 17956130 Free PMC article.
-
Control of depolarization-evoked presynaptic neurotransmitter release by Cav2.1 calcium channel: old story, new insights.Channels (Austin). 2010 Nov-Dec;4(6):431-3. doi: 10.4161/chan.4.6.13613. Epub 2010 Nov 1. Channels (Austin). 2010. PMID: 20935476 Free PMC article.
-
Is it zippered? Does it flare? That darn complexin clamping SNARE.Biophys J. 2013 Aug 20;105(4):835-6. doi: 10.1016/j.bpj.2013.07.007. Biophys J. 2013. PMID: 23972833 Free PMC article. No abstract available.
References
-
- Mayer, A. 2002. Membrane fusion in eukaryotic cells. Annu. Rev. Cell Dev. Biol. 18:289–314. - PubMed
-
- Jahn, R., T. Lang, and T. C. Sudhof. 2003. Membrane fusion. Cell. 112:519–533. - PubMed
-
- Martin, T. F. 2003. Tuning exocytosis for speed: fast and slow modes. Biochim. Biophys. Acta. 1641:157–165. - PubMed
-
- Weber, T., B. V. Zemelman, J. A. McNew, B. Westermann, M. Gmachl, F. Parlati, T. H. Sollner, and J. E. Rothman. 1998. SNAREpins: minimal machinery for membrane fusion. Cell. 92:759–772. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

