Molecular identification and reconstitution of depolarization-induced exocytosis monitored by membrane capacitance
- PMID: 16150968
- PMCID: PMC1367000
- DOI: 10.1529/biophysj.105.064642
Molecular identification and reconstitution of depolarization-induced exocytosis monitored by membrane capacitance
Abstract
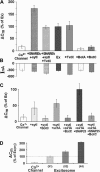
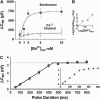
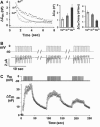
Regulated exocytosis of neurotransmitters at synapses is fast and tightly regulated. It is unclear which proteins constitute the "minimal molecular machinery" for this process. Here, we show that a novel technique of capacitance monitoring combined with heterologous protein expression can be used to reconstitute exocytosis that is fast (<0.5 s) and triggered directly by membrane depolarization in Xenopus oocytes. Testing synaptic proteins, voltage-gated Ca2+ channels, and using botulinum and tetanus neurotoxins established that the expression of a Ca2+ channel together with syntaxin 1A, SNAP-25, and synaptotagmin was sufficient and necessary for the reconstitution of depolarization-induced exocytosis. Similar to synaptic exocytosis, the reconstituted release was sensitive to neurotoxins, modulated by divalent cations (Ca2+, Ba2+, and Sr2+) or channel (Lc-, N-type), and depended nonlinearly on divalent cation concentration. Because of its improved speed, native trigger, and great experimental versatility, this reconstitution assay provides a novel, promising tool to study synaptic exocytosis.
Figures


References
-
- Mayer, A. 2002. Membrane fusion in eukaryotic cells. Annu. Rev. Cell Dev. Biol. 18:289–314. - PubMed
-
- Jahn, R., T. Lang, and T. C. Sudhof. 2003. Membrane fusion. Cell. 112:519–533. - PubMed
-
- Martin, T. F. 2003. Tuning exocytosis for speed: fast and slow modes. Biochim. Biophys. Acta. 1641:157–165. - PubMed
-
- Weber, T., B. V. Zemelman, J. A. McNew, B. Westermann, M. Gmachl, F. Parlati, T. H. Sollner, and J. E. Rothman. 1998. SNAREpins: minimal machinery for membrane fusion. Cell. 92:759–772. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

