1H-NMR study of the effect of temperature through reversible unfolding on the heme pocket molecular structure and magnetic properties of aplysia limacina cyano-metmyoglobin
- PMID: 16150970
- PMCID: PMC1366980
- DOI: 10.1529/biophysj.105.062398
1H-NMR study of the effect of temperature through reversible unfolding on the heme pocket molecular structure and magnetic properties of aplysia limacina cyano-metmyoglobin
Abstract
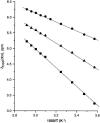
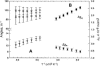
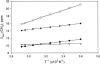
Two-dimensional 1H NMR spectroscopy over a range of temperature through thermal unfolding has been applied to the low-spin, ferric cyanide complex of myoglobin from Aplysia limacina to search for intermediates in the unfolding and to characterize the effect of temperature on the magnetic properties and electronic structure of the heme iron. The observation of strictly linear behavior from 5 to 80 C degrees through the unfolding transition for all hyperfine-shifted resonances indicates the absence of significant populations of intermediate states to the cooperative unfolding with Tm approximately 80 degrees C. The magnetic anisotropies and orientation of the magnetic axes for the complete range of temperatures were also determined for the complex. The anisotropies have very similar magnitudes, and exhibit the expected characteristic temperature dependence, previously observed in the isoelectronic sperm whale myoglobin complex. In contrast to sperm whale Mb, where the orientation of the magnetic axis was completely temperature-independent, the tilt of the major magnetic axis, which correlates with the Fe-CN tilt, decreases at high temperature in Aplysia limacina Mb, indicating a molecular structure that is conserved with temperature, although more plastic than that of sperm whale Mb. The pattern of contact shifts reflects a conserved Fe-His(F8) bond and pi-spin delocalization into the heme, as expected for the orientation of the axial His imidazole.
Figures





References
-
- Antonini, E., and M. Brunori. 1971. Hemoglobin and Myoglobin and Their Reactions with Ligands. Elsevier, North-Holland Publishing, Amsterdam, The Netherlands. 40–54.
-
- Dickerson, R. E., and I. Geis. 1983. Hemoglobin: Structure, Function, Evolution and Pathology. Benjamin-Cummings, Menlo Park, CA.
-
- Griko, Y. V., P. L. Privalov, S. Y. Venyaminos, and V. P. Kutyshenko. 1988. Thermodynamic study of apomyoglobin structure. J. Mol. Biol. 202:127–138. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

