Epoxide formation on the aromatic B ring of flavanone by biphenyl dioxygenase of Pseudomonas pseudoalcaligenes KF707
- PMID: 16151125
- PMCID: PMC1214616
- DOI: 10.1128/AEM.71.9.5354-5361.2005
Epoxide formation on the aromatic B ring of flavanone by biphenyl dioxygenase of Pseudomonas pseudoalcaligenes KF707
Abstract
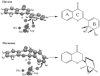
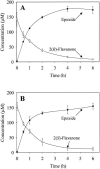
Prokaryotic dioxygenase is known to catalyze aromatic compounds into their corresponding cis-dihydrodiols without the formation of an epoxide intermediate. Biphenyl dioxygenase from Pseudomonas pseudoalcaligenes KF707 showed novel monooxygenase activity by converting 2(R)- and 2(S)-flavanone to their corresponding epoxides (2-(7-oxabicyclo[4.1.0]hepta-2,4-dien-2-yl)-2, 3-dihydro-4H-chromen-4-one), whereby the epoxide bond was formed between C2' and C3' on the B ring of the flavanone. The enzyme also converted 6-hydroxyflavanone and 7-hydroxyflavanone, which do not contain a hydroxyl group on the B-ring, to their corresponding epoxides. In a previous report (S.-Y. Kim, J. Jung, Y. Lim, J.-H. Ahn, S.-I. Kim, and H.-G. Hur, Antonie Leeuwenhoek 84:261-268, 2003), however, we found that the same enzyme showed dioxygenase activity toward flavone, resulting in the production of flavone cis-2',3'-dihydrodiol. Extensive structural identification of the metabolites of flavanone by using high-pressure liquid chromatography, liquid chromatography/mass spectrometry, and nuclear magnetic resonance confirmed the presence of an epoxide functional group on the metabolites. Epoxide formation as the initial activation step of aromatic compounds by oxygenases has been reported to occur only by eukaryotic monooxygenases. To the best of our knowledge, biphenyl dioxygenase from P. pseudoalcaligenes KF707 is the first prokaryotic enzyme detected that can produce an epoxide derivative on the aromatic ring structure of flavanone.
Figures



Similar articles
-
Cis-2', 3'-dihydrodiol production on flavone B-ring by biphenyl dioxygenase from Pseudomonas pseudoalcaligenes KF707 expressed in Escherichia coli.Antonie Van Leeuwenhoek. 2003;84(4):261-8. doi: 10.1023/a:1026081824334. Antonie Van Leeuwenhoek. 2003. PMID: 14574103
-
Absolute configuration-dependent epoxide formation from isoflavan-4-ol stereoisomers by biphenyl dioxygenase of Pseudomonas pseudoalcaligenes strain KF707.Appl Microbiol Biotechnol. 2011 Mar;89(6):1773-82. doi: 10.1007/s00253-010-2989-1. Epub 2010 Nov 10. Appl Microbiol Biotechnol. 2011. PMID: 21063701
-
Flavonoids biotransformation by bacterial non-heme dioxygenases, biphenyl and naphthalene dioxygenase.Appl Microbiol Biotechnol. 2011 Jul;91(2):219-28. doi: 10.1007/s00253-011-3334-z. Epub 2011 May 28. Appl Microbiol Biotechnol. 2011. PMID: 21626021 Review.
-
Time-dependent density functional theory-assisted absolute configuration determination of cis-dihydrodiol metabolite produced from isoflavone by biphenyl dioxygenase.Anal Biochem. 2010 Feb 1;397(1):29-36. doi: 10.1016/j.ab.2009.10.020. Epub 2009 Oct 23. Anal Biochem. 2010. PMID: 19854147
-
Dioxygenase- and monooxygenase-catalysed synthesis of cis-dihydrodiols, catechols, epoxides and other oxygenated products.Biotechnol Lett. 2008 Nov;30(11):1879-91. doi: 10.1007/s10529-008-9791-5. Epub 2008 Jul 9. Biotechnol Lett. 2008. PMID: 18612597 Review.
Cited by
-
Remarkable ability of Pandoraea pnomenusa B356 biphenyl dioxygenase to metabolize simple flavonoids.Appl Environ Microbiol. 2012 May;78(10):3560-70. doi: 10.1128/AEM.00225-12. Epub 2012 Mar 16. Appl Environ Microbiol. 2012. PMID: 22427498 Free PMC article.
-
Bioactive Components and Health Benefits of Saskatoon Berry.J Diabetes Res. 2020 May 14;2020:3901636. doi: 10.1155/2020/3901636. eCollection 2020. J Diabetes Res. 2020. PMID: 32509879 Free PMC article. Review.
-
Drug activity screening based on microsomes-hydrogel system in predicting metabolism induced antitumor effect of oroxylin A.Sci Rep. 2016 Feb 24;6:21604. doi: 10.1038/srep21604. Sci Rep. 2016. PMID: 26905263 Free PMC article.
-
Predominant Biphenyl Dioxygenase From Legacy Polychlorinated Biphenyl (PCB)-Contaminated Soil Is a Part of Unusual Gene Cluster and Transforms Flavone and Flavanone.Front Microbiol. 2021 Oct 14;12:644708. doi: 10.3389/fmicb.2021.644708. eCollection 2021. Front Microbiol. 2021. PMID: 34721309 Free PMC article.
-
Chemical Aspects of Gut Metabolism of Flavonoids.Metabolites. 2019 Jul 10;9(7):136. doi: 10.3390/metabo9070136. Metabolites. 2019. PMID: 31295867 Free PMC article. Review.
References
-
- Bax, A., and R. Freeman. 1981. A simple method for suppressing dispersion-mode contributions in NMR spectra: the “pseudo echo.” J. Magn. Reson. 43:333-338.
-
- Bax, A., R. Griffey, and B. Hawkins. 1983. Correlation of proton and nitrogen-15 chemical shifts by multiple quantum NMR. J. Magn. Reson. 55:301-315.
-
- Bax, A., and M. Summers. 1986. 1H and 13C assignments from sensitivity-enhanced detection of heteronuclear multiple-bond connectivity by 2D multiple quantum NMR. J. Am. Chem. Soc. 108:2093-2094.
-
- Boyd, D. R., N. D. Sharma, and C. C. Allen. 2001. Aromatic dioxygenases: molecular biocatalysis and applications. Curr. Opin. Biotechnol. 12:564-573. - PubMed
-
- Carredano, E., A. Karlsson, B. Kauppi, D. Choudhury, R. E. Parales, J. V. Parales, K. Lee, D. T. Gibson, H. Eklund, and S. Ramaswamy. 2000. Substrate binding site of naphthalene 1,2-dioxygenase: functional implications of indole binding. J. Mol. Biol. 296:701-712. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

