Calcineurin-binding protein Cbp1 directs the specificity of calcineurin-dependent hyphal elongation during mating in Cryptococcus neoformans
- PMID: 16151246
- PMCID: PMC1214203
- DOI: 10.1128/EC.4.9.1526-1538.2005
Calcineurin-binding protein Cbp1 directs the specificity of calcineurin-dependent hyphal elongation during mating in Cryptococcus neoformans
Abstract
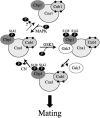
Mating and virulence of the human fungal pathogen Cryptococcus neoformans are controlled by calcineurin, a serine-threonine-specific calcium-activated phosphatase that is the target of the immunosuppressive drugs cyclosporine A and FK506. In previous studies, a calcineurin binding protein (Cbp1, Rcn1, Dscr1/Csp1-3/MCIP1-3) that is conserved from yeasts to humans has been identified, but whether this protein functions to regulate calcineurin activity or facilitate calcineurin function as a signaling effector has been unclear. Here we show that, like calcineurin, Cbp1 is required for mating in C. neoformans. By contrast, Cbp1 plays no role in promoting calcineurin-dependent growth at 37 degrees C and is not essential for haploid fruiting. Site-directed mutagenesis studies provide evidence that tandem phosphorylation and dephosphorylation of two serine residues in the conserved SP repeat motif are critical for Cbp1 function. Epistasis analysis supports models in which Cbp1 functions coordinately with calcineurin to direct hyphal elongation during mating. Taken together, these findings provide insights into the roles of Cbp1 as an accessory subunit or effector of calcineurin-specific signaling pathways, which may be features conserved among the calcipressins to govern calcineurin signaling in immune cells, cardiomyocytes, and neurons of multicellular eukaryotes.
Figures





Similar articles
-
Calcineurin is required for hyphal elongation during mating and haploid fruiting in Cryptococcus neoformans.EMBO J. 2001 Mar 1;20(5):1020-32. doi: 10.1093/emboj/20.5.1020. EMBO J. 2001. PMID: 11230126 Free PMC article.
-
Identification and characterization of a highly conserved calcineurin binding protein, CBP1/calcipressin, in Cryptococcus neoformans.EMBO J. 2000 Jul 17;19(14):3618-29. doi: 10.1093/emboj/19.14.3618. EMBO J. 2000. PMID: 10899116 Free PMC article.
-
Phospholipid-binding protein Cts1 controls septation and functions coordinately with calcineurin in Cryptococcus neoformans.Eukaryot Cell. 2003 Oct;2(5):1025-35. doi: 10.1128/EC.2.5.1025-1035.2003. Eukaryot Cell. 2003. PMID: 14555485 Free PMC article.
-
Signal transduction pathways regulating differentiation and pathogenicity of Cryptococcus neoformans.Fungal Genet Biol. 1998 Oct;25(1):1-14. doi: 10.1006/fgbi.1998.1079. Fungal Genet Biol. 1998. PMID: 9806801 Review.
-
Signal transduction cascades regulating mating, filamentation, and virulence in Cryptococcus neoformans.Curr Opin Microbiol. 1999 Aug;2(4):358-62. doi: 10.1016/S1369-5274(99)80063-0. Curr Opin Microbiol. 1999. PMID: 10458985 Review.
Cited by
-
Elucidation of the calcineurin-Crz1 stress response transcriptional network in the human fungal pathogen Cryptococcus neoformans.PLoS Genet. 2017 Apr 4;13(4):e1006667. doi: 10.1371/journal.pgen.1006667. eCollection 2017 Apr. PLoS Genet. 2017. PMID: 28376087 Free PMC article.
-
Dissecting the Roles of the Calcineurin Pathway in Unisexual Reproduction, Stress Responses, and Virulence in Cryptococcus deneoformans.Genetics. 2018 Feb;208(2):639-653. doi: 10.1534/genetics.117.300422. Epub 2017 Dec 12. Genetics. 2018. PMID: 29233811 Free PMC article.
-
The regulation of the sulfur amino acid biosynthetic pathway in Cryptococcus neoformans: the relationship of Cys3, Calcineurin, and Gpp2 phosphatases.Sci Rep. 2019 Aug 15;9(1):11923. doi: 10.1038/s41598-019-48433-5. Sci Rep. 2019. PMID: 31417135 Free PMC article.
-
Calcineurin regulation in fungi and beyond.Eukaryot Cell. 2008 Feb;7(2):177-86. doi: 10.1128/EC.00326-07. Epub 2007 Dec 7. Eukaryot Cell. 2008. PMID: 18065652 Free PMC article. Review. No abstract available.
-
RNA sequencing reveals an additional Crz1-binding motif in promoters of its target genes in the human fungal pathogen Candida albicans.Cell Commun Signal. 2020 Jan 3;18(1):1. doi: 10.1186/s12964-019-0473-9. Cell Commun Signal. 2020. PMID: 31900175 Free PMC article.
References
-
- Alspaugh, J. A., R. C. Davidson, and J. Heitman. 2000. Morphogenesis of Cryptococcus neoformans, p. 217-238. In J. F. Ernst and A. Schmidt (ed.), Dimorphism in human pathogenic and apathogenic yeasts, vol. 5. Karger, Basel, Switzerland. - PubMed
-
- Chang, H. Y., K. Takei, A. M. Sydor, T. Born, F. Rusnak, and D. G. Jay. 1995. Asymmetric retraction of growth cone filopodia following focal inactivation of calcineurin. Nature 376:686-690. - PubMed
-
- Cho, Y. J., M. Abe, S. Y. Kim, and Y. Sato. 2005. Raf-1 is a binding partner of DSCR1. Arch. Biochem. Biophys. 439:121-128. - PubMed
-
- Davidson, R. C., J. R. Blankenship, P. R. Kraus, M. de Jesus Berrios, C. M. Hull, C. D'Souza, P. Wang, and J. Heitman. 2002. A PCR-based strategy to generate integrative targeting alleles with large regions of homology. Microbiology 148:2607-2615. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

