Apoptosis modulates protective immunity to the pathogenic fungus Histoplasma capsulatum
- PMID: 16151533
- PMCID: PMC1199552
- DOI: 10.1172/JCI25365
Apoptosis modulates protective immunity to the pathogenic fungus Histoplasma capsulatum
Abstract
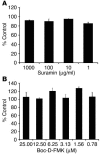
Pathogen-induced apoptosis of lymphocytes is associated with increased susceptibility to infection. In this study, we determined whether apoptosis influenced host resistance to the fungus Histoplasma capsulatum. The level of apoptotic leukocytes progressively increased in the lungs of naive and immune mice during the course of H. capsulatum infection. T cells constituted the dominant apoptotic population. Apoptosis was diminished in H. capsulatum-infected gld/gld and TNF-alpha-deficient mice; concomitantly, the fungal burden exceeded that of controls. Treatment of naive and H. capsulatum-immune mice with caspase inhibitors decreased apoptosis but markedly enhanced the severity of infection. Administration of a proapoptotic dose of suramin diminished the fungal burden. The increased burden in recipients of a caspase inhibitor was associated with elevations in IL-4 and IL-10 levels. In the absence of either of these cytokines, caspase inhibition suppressed apoptosis but did not increase the fungal burden. Thus, apoptosis is a critical element of protective immunity to H. capsulatum. Production of IL-4 and IL-10 is markedly elevated when apoptosis is inhibited, and the release of these cytokines exacerbates the severity of infection.
Figures




References
-
- Deepe, G.S., Jr. 2000. Histoplasma capsulatum. In Principles and practice of infectious diseases. 5th edition. G.L. Mandell, J.E. Bennett, and R. Dolin, editors. Churchill Livingstone. Philadelphia, Pennsylvania, USA. 2718–2732.
-
- Allendorfer R, Brunner GD, Deepe GS., Jr Complex requirements for nascent and memory immunity in pulmonary histoplasmosis. J. Immunol. 1999;162:7389–7396. - PubMed
-
- Deepe GS., Jr Role of CD8+ T cells in host resistance to systemic infection with Histoplasma capsulatum in mice. J. Immunol. 1994;152:3491–3500. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

