Humanization of a recombinant monoclonal antibody to produce a therapeutic HER dimerization inhibitor, pertuzumab
- PMID: 16151804
- PMCID: PMC11030689
- DOI: 10.1007/s00262-005-0058-x
Humanization of a recombinant monoclonal antibody to produce a therapeutic HER dimerization inhibitor, pertuzumab
Abstract
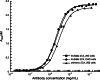
Dimerization is essential for activity of human epidermal growth factor receptors (HER1/EGFR, HER2/ErbB2, HER3/ErbB3, and ErbB4) and mediates intracellular signaling events leading to cancer cell proliferation, survival, and resistance to therapy. HER2 is the preferred dimerization partner. Activation of HER signaling pathways may be blocked by inhibition of dimer formation using a monoclonal antibody (MAb) directed against the dimerization domain of HER2. The murine MAb 2C4 that specifically binds the HER2 dimerization domain was cloned as a chimeric antibody, humanized using a computer-generated model to guide framework substitutions, and variants were tested as Fabs. Pharmacokinetics and toxicology were evaluated in rodents and cynomolgus monkeys. Cloning the variable domains of MAb 2C4 into a vector containing human kappa and CH1 domains allowed construction of a mouse-human chimeric Fab. DNA sequencing of the chimeric clone permitted identification of CDR residues. The full-length IgG1 of variant F-10 was equivalent in binding to chimeric IgG1 and was designated pertuzumab (rhuMAb 2C4; Omnitarg). Pertuzumab pharmacokinetics was best described by a two-compartment model with a distribution phase of <1 day, terminal half-life of approximately 10 days, and volume of distribution of approximately 40 mL/kg that approximates serum volume. With the exception of diarrhea, pertuzumab was generally well tolerated in cynomolgus monkeys. Pertuzumab, a recombinant humanized IgG1 MAb, is the first of a new class of agents known as HER dimerization inhibitors. Inhibition of HER dimerization may be an effective anticancer strategy in tumors with either normal or elevated expression of HER2.
Figures


References
-
- Agus DB, Akita RW, Fox WD, Lofgren JA, Higgins B, Maiese K, Scher HI, Sliwkowski MX. A potential role for activated HER-2 in prostate cancer. Semin Oncol. 2000;27:76–83. - PubMed
-
- Agus DB, Gordon MS, Taylor C, Natale RB, Karlan B, Mendelson DS, Press MF, Allison DE, Sliwkowski MX, Lieberman G, Kelsey SM, Fyfe G. Phase I clinical study of pertuzumab, a novel HER dimerization inhibitor, in patients with advanced cancer. J Clin Oncol. 2005;23:2534–2543. doi: 10.1200/JCO.2005.03.184. - DOI - PubMed
-
- Alimandi M, Romano A, Curia MC, Muraro R, Fedi P, Aaronson SA, Di Fiore PP, Kraus MH. Cooperative signaling of ErbB3 and ErbB2 in neoplastic transformation and human mammary carcinomas. Oncogene. 1995;10:1813–1821. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

