The phenotypic spectrum in patients with arginine to cysteine mutations in the COL2A1 gene
- PMID: 16155195
- PMCID: PMC2564515
- DOI: 10.1136/jmg.2005.035717
The phenotypic spectrum in patients with arginine to cysteine mutations in the COL2A1 gene
Abstract
Background: The majority of COL2A1 missense mutations are substitutions of obligatory glycine residues in the triple helical domain. Only a few non-glycine missense mutations have been reported and among these, the arginine to cysteine substitutions predominate.
Objective: To investigate in more detail the phenotype resulting from arginine to cysteine mutations in the COL2A1 gene.
Methods: The clinical and radiographic phenotype of all patients in whom an arginine to cysteine mutation in the COL2A1 gene was identified in our laboratory, was studied and correlated with the abnormal genotype. The COL2A1 genotyping involved DHPLC analysis with subsequent sequencing of the abnormal fragments.
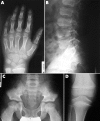
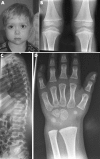
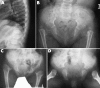
Results: Six different mutations (R75C, R365C, R519C, R704C, R789C, R1076C) were found in 11 unrelated probands. Each mutation resulted in a rather constant and site-specific phenotype, but a perinatally lethal disorder was never observed. Spondyloarthropathy with normal stature and no ocular involvement were features of patients with the R75C, R519C, or R1076C mutation. Short third and/or fourth toes was a distinguishing feature of the R75C mutation and brachydactyly with enlarged finger joints a key feature of the R1076C substitution. Stickler dysplasia with brachydactyly was observed in patients with the R704C mutation. The R365C and R789C mutations resulted in classic Stickler dysplasia and spondyloepiphyseal dysplasia congenita (SEDC), respectively.
Conclusions: Arginine to cysteine mutations are rather infrequent COL2A1 mutations which cause a spectrum of phenotypes including classic SEDC and Stickler dysplasia, but also some unusual entities that have not yet been recognised and described as type II collagenopathies.
Conflict of interest statement
Competing interests: none declared
References
-
- Spranger J, Winterpacht A, Zabel B. The type II collagenopathies: a spectrum of chondrodysplasias. Eur J Pediatr 1994153(2)56–65. - PubMed
-
- Zankl A, Neumann L, Ignatius J, Nikkels P, Schrander‐Stumpel C, Mortier G, Omran H, Wright M, Hilbert K, Bonafe L, Spranger J, Zabel B, Superti‐Furga A. Dominant negative mutations in the C‐propeptide of COL2A1 cause platyspondylic lethal skeletal dysplasia, torrance type, and define a novel subfamily within the type 2 collagenopathies. Am J Med Genet A 2005133(1)61–67. - PubMed
-
- Kuivaniemi H, Tromp G, Prockop D J. Mutations in fibrillar collagens (types I, II, III, and XI), fibril‐associated collagen (type IX), and network‐forming collagen (type X) cause a spectrum of diseases of bone, cartilage, and blood vessels. Hum Mutat 19979(4)300–315. - PubMed
-
- Bleasel J F, Holderbaum D, Mallock V, Haqqi T M, Williams H J, Moskowitz R W. Hereditary osteoarthritis with mild spondyloepiphyseal dysplasia: are there “hot spots” on COL2A1? J Rheumatol 199623(9)1594–1598. - PubMed
-
- Kielty C M, Grant M E. The collagen family: structure, assembly, and organization in the extracellular matrix. In: Royce PM, Steinmann B, eds. Connective tissue and its heritable disorders. New York: Wiley‐Liss, 2002159–222.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources