Motor-induced transcription but sensory-regulated translation of ZENK in socially interactive songbirds
- PMID: 16155900
- PMCID: PMC4264577
- DOI: 10.1002/neu.20187
Motor-induced transcription but sensory-regulated translation of ZENK in socially interactive songbirds
Abstract
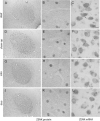
The ZENK gene, depending upon singing activity, is transcribed within all the telencephalic nuclei controlling vocal behavior in songbirds. We show here that singing by deafened or completely isolated adult zebra finches induced high levels of ZENK transcription. This mRNA however, was not translated into high levels of ZENK protein. Instead, high levels of singing-driven ZENK protein translation were found in socially interactive birds. This dissociation between ZENK mRNA and ZENK protein was regionally specific to the robust nucleus of the arcopallium (RA), a region that is well known for its control of vocal-motor behavior in birds. Our results suggest cooperation between motor and sensory processes for regulating mRNA induction and subsequent protein synthesis in socially active songbirds.
Figures



References
-
- Arnold AP. Quantitative analysis of sex differences in hormone accumulation in the zebra finch brain: methodological and theoretical issues. J Comp Neurol. 1980;189:421–426. - PubMed
-
- [2005 Apr 2];Avisoft Bioacoustics [Internet] Germany: Avisoft Bio-acoustics. 2005 Available from: http://www.avisoft.de./
-
- Bartel DP, Chen CZ. Micromanagers of gene expression: the potentially widespread influence of metazoan microRNAs. Nat Rev Genet. 2004;5:396–400. - PubMed
-
- Bottjer SW, Miesner EA, Arnold AP. Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science. 1984;224:901–903. - PubMed
-
- Brainard MS, Doupe AJ. Interruption of a basal ganglia-forebrain circuit prevents plasticity of learned vocalizations. Nature. 2000a;404:762–766. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

