Modulatory effects of plant phenols on human multidrug-resistance proteins 1, 4 and 5 (ABCC1, 4 and 5)
- PMID: 16156793
- PMCID: PMC1350838
- DOI: 10.1111/j.1742-4658.2005.04888.x
Modulatory effects of plant phenols on human multidrug-resistance proteins 1, 4 and 5 (ABCC1, 4 and 5)
Abstract
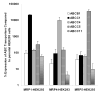
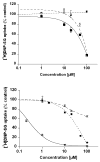
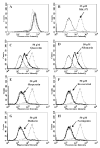
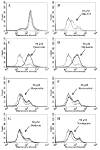
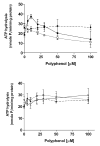
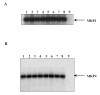
Plant flavonoids are polyphenolic compounds, commonly found in vegetables, fruits and many food sources that form a significant portion of our diet. These compounds have been shown to interact with several ATP-binding cassette transporters that are linked with anticancer and antiviral drug resistance and, as such, may be beneficial in modulating drug resistance. This study investigates the interactions of six common polyphenols; quercetin, silymarin, resveratrol, naringenin, daidzein and hesperetin with the multidrug-resistance-associated proteins, MRP1, MRP4 and MRP5. At nontoxic concentrations, several of the polyphenols were able to modulate MRP1-, MRP4- and MRP5-mediated drug resistance, though to varying extents. The polyphenols also reversed resistance to NSC251820, a compound that appears to be a good substrate for MRP4, as predicted by data-mining studies. Furthermore, most of the polyphenols showed direct inhibition of MRP1-mediated [3H]dinitrophenyl S-glutathione and MRP4-mediated [3H]cGMP transport in inside-out vesicles prepared from human erythrocytes. Also, both quercetin and silymarin were found to inhibit MRP1-, MRP4- and MRP5-mediated transport from intact cells with high affinity. They also had significant effects on the ATPase activity of MRP1 and MRP4 without having any effect on [32P]8-azidoATP[alphaP] binding to these proteins. This suggests that these flavonoids most likely interact at the transporter's substrate-binding sites. Collectively, these results suggest that dietary flavonoids such as quercetin and silymarin can modulate transport activities of MRP1, -4 and -5. Such interactions could influence bioavailability of anticancer and antiviral drugs in vivo and thus, should be considered for increasing efficacy in drug therapies.
Figures




References
-
- Allen JD, Brinkhuis RF, van Deemter L, Wijnholds J, Schinkel AH. Extensive contribution of the multidrug transporters P-glycoprotein and Mrp1 to basal drug resistance. Cancer Res. 2000;60:5761–5766. - PubMed
-
- Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. - PubMed
-
- Borst P, Oude-Elferink R. Mammalian ABC Transporters in health and disease. Annual Review of Biochemistry. 2002;71:537–592. - PubMed
-
- Cole SP, Sparks KE, Fraser K, Loe DW, Grant CE, Wilson GM, Deeley RG. Pharmacological characterization of multidrug resistant MRP-transfected human tumor cells. Cancer Res. 1994;54:5902–10. - PubMed
-
- Reid G, Wielinga P, Zelcer N, De Haas M, Van Deemter L, Wijnholds J, Balzarini J, Borst P. Characterization of the transport of nucleoside analog drugs by the human multidrug resistance proteins MRP4 and MRP5. Mol Pharmacol. 2003;63:1094–103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

