Maternal plasma osteoprotegerin concentration in normal pregnancy
- PMID: 16157103
- PMCID: PMC1351230
- DOI: 10.1016/j.ajog.2005.06.051
Maternal plasma osteoprotegerin concentration in normal pregnancy
Abstract
Objective: Pregnancy is associated with major changes in calcium metabolism because the neonatal skeleton contains approximately 30 g of calcium, which are largely deposited in the third trimester. Osteoprotegerin (OPG) acts as a decoy receptor for the "Receptor Activator of Nuclear Factor-kappaB Ligand" (RANKL), which is an essential factor for bone remodeling. This study was conducted to determine whether there were changes in maternal plasma OPG concentration during normal pregnancy.
Study design: A cross-sectional study was performed in 433 patients of reproductive age (40 nonpregnant and 393 pregnant). Pregnant patients were classified into 4 groups according to gestational age: group 1: 11 to 14 weeks (n = 100); group 2: 15 to 18 weeks (n = 99); group 3: 27 to 30 weeks (n = 100); and group 4: 37 to 42 weeks (n = 94). Plasma OPG concentrations were measured with the use of a sensitive and specific immunoassay. Nonparametric statistics were used for analysis.
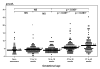
Results: OPG was detected in the plasma of all women tested. The median OPG concentration was significantly higher in term patients than in those in early pregnancy (median: 6.63 pmol/L [range: 1.57-25.57] vs median: 3.98 pmol/L [range: 0.41-13.71], P < .001). There was no significant difference in plasma OPG concentrations between nonpregnant women and those in groups 1 or 2 (nonpregnant women median: 3.86 pmol/L [range: 1.64-15.29] vs group 1 median: 3.98 pmol/L [range: 0.41-13.71] vs group 2 median: 3.87 pmol/L [range: 1.14-69.83], P = .75).
Conclusion: The median maternal plasma OPG concentration is higher in the third trimester than in the first trimester of pregnancy. OPG may be involved in the regulation of bone turnover during pregnancy.
Figures

References
-
- Givens MH, Macy IC. The chemical composition of the human fetus. J Biol Chem. 1933;102:7–17.
-
- Pitkin RM. Calcium metabolism in pregnancy and the perinatal period: a review. Am J Obstet Gynecol. 1985;151:99–109. - PubMed
-
- Saxe A, Dean S, Gibson G, Pandian MR, Levy J. Parathyroid hormone and parathyroid hormone-related peptide in venous umbilical cord blood of healthy neonates. J Perinat Med. 1997;25:288–91. - PubMed
-
- Cross NA, Hillman LS, Allen SH, Krause GF, Vieira NE. Calcium homeostasis and bone metabolism during pregnancy, lactation, and postweaning: a longitudinal study. Am J Clin Nutr. 1995;61:514–23. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

