Tacrolimus versus ciclosporin as primary immunosuppression for kidney transplant recipients: meta-analysis and meta-regression of randomised trial data
- PMID: 16157605
- PMCID: PMC1246079
- DOI: 10.1136/bmj.38569.471007.AE
Tacrolimus versus ciclosporin as primary immunosuppression for kidney transplant recipients: meta-analysis and meta-regression of randomised trial data
Abstract
Objective: To compare the positive and negative effects of tacrolimus and ciclosporin as initial treatment for renal transplant recipients.
Design: Systematic review.
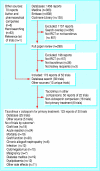
Data sources and study selection: Reports of comparative randomised trials of tacrolimus and ciclosporin identified by searches of Medline, Embase, the Cochrane Register of Controlled Trials, the Cochrane Renal Group Specialist Register, and conference proceedings.
Data extraction and synthesis: Two reviewers assessed trials for eligibility and quality and extracted data independently. Data were synthesised (random effects model) and results expressed as relative risk (RR), with values < 1 favouring tacrolimus. Subgroup analysis and meta-regression were used to examine potential effect modification by differences in trial design and immunosuppressive co-interventions.
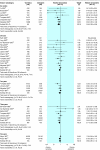
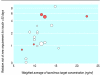
Results: 123 reports from 30 trials (4102 patients) were included. At six months, graft loss was significantly reduced in tacrolimus treated recipients (RR = 0.56, 95% confidence interval 0.36 to 0.86), and this effect persisted up to three years. The relative reduction in graft loss with tacrolimus diminished with higher concentrations of tacrolimus (P = 0.04) but did not vary with ciclosporin formulation (P = 0.97) or ciclosporin concentration (P = 0.38). At one year, tacrolimus treated patients had less acute rejection (RR = 0.69, 0.60 to 0.79) and less steroid resistant rejection (RR = 0.49, 0.37 to 0.64) but more diabetes mellitus requiring insulin (RR = 1.86, 1.11 to 3.09), tremor, headache, diarrhoea, dyspepsia, and vomiting. The relative excess of diabetes increased with higher concentrations of tacrolimus (P = 0.003). Ciclosporin treated recipients had significantly more constipation and cosmetic side effects. No differences were seen in infection or malignancy.
Conclusions: Treating 100 recipients with tacrolimus instead of ciclosporin for the first year after transplantation avoids 12 patients having acute rejection and two losing their graft but causes an extra five patients to develop insulin dependent diabetes. Optimal drug choice may vary between patients.
Figures



References
-
- Chadban S. Transplantation: ANZDATA registry report 2003. Adelaide: Australian and New Zealand Dialysis And Transplant Registry, 2003: 65.
-
- Immunosuppression: 2004 annual report of the U.S. Organ Procurement and Transplantation Network and the scientific registry of transplant recipients: transplant data 1994-2003. Ann Arbor, MI: HHS/HRSA/OSP/DOT and UNOS, 2004.
-
- Hong JC, Kahan BD. Immunosuppressive agents in organ transplantation: past, present, and future. Semin Nephrol 2000;20: 108-25. - PubMed
-
- Meier-Kriesche HU, Schold JD, Srinivas TR, Kaplan B. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant 2004;4: 378-83. - PubMed
-
- Pascual M, Theruvath T, Kawai T, Tolkoff-Rubin N, Cosimi AB. Strategies to improve long-term outcomes after renal transplantation. N Engl J Med 2002;346: 580-90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
