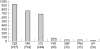Malformation risks of antiepileptic drugs in pregnancy: a prospective study from the UK Epilepsy and Pregnancy Register
- PMID: 16157661
- PMCID: PMC2077578
- DOI: 10.1136/jnnp.2005.074203
Malformation risks of antiepileptic drugs in pregnancy: a prospective study from the UK Epilepsy and Pregnancy Register
Abstract
Objective: To assess the relative risk of major congenital malformation (MCM) from in utero exposure to antiepileptic drug (AEDs).
Methods: Prospective data collected by the UK Epilepsy and Pregnancy Register were analysed. The presence of MCMs recorded within the first three months of life was the main outcome measure.
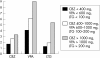
Results: Full outcome data were collected on 3607 cases. The overall MCM rate for all AED exposed cases was 4.2% (95% confidence interval (CI), 3.6% to 5.0%). The MCM rate was higher for polytherapy (6.0%) (n = 770) than for monotherapy (3.7%) (n = 2598) (crude odds ratio (OR) = 1.63 (p = 0.010), adjusted OR = 1.83 (p = 0.002)). The MCM rate for women with epilepsy who had not taken AEDs during pregnancy (n = 239) was 3.5% (1.8% to 6.8%). The MCM rate was greater for pregnancies exposed only to valproate (6.2% (95% CI, 4.6% to 8.2%) than only to carbamazepine (2.2% (1.4% to 3.4%) (OR = 2.78 (p<0.001); adjusted OR = 2.97 (p<0.001)). There were fewer MCMs for pregnancies exposed only to lamotrigine than only to valproate. A positive dose response for MCMs was found for lamotrigine (p = 0.006). Polytherapy combinations containing valproate carried a higher risk of MCM than combinations not containing valproate (OR = 2.49 (1.31 to 4.70)).
Conclusions: Only 4.2% of live births to women with epilepsy had an MCM. The MCM rate for polytherapy exposure was greater than for monotherapy exposure. Polytherapy regimens containing valproate had significantly more MCMs than those not containing valproate. For monotherapy exposures, carbamazepine was associated with the lowest risk of MCM.
Conflict of interest statement
Competing interests: JC, AR, LP, PM, RW, BI, and JM have attended meetings with the support of various pharmaceutical companies, including Glaxo‐Smith‐Kline. JC, LP, PM, and JM have given lectures at the bequest of pharmaceutical companies, including Glaxo‐Smith‐Kline, for which they have received honoraria. IR and CMcG have declared no conflicts of interest.
Comment in
-
Major congenital malformations and antiepileptic drugs: prospective observations.J Neurol Neurosurg Psychiatry. 2006 Feb;77(2):145. doi: 10.1136/jnnp.2005.079376. J Neurol Neurosurg Psychiatry. 2006. PMID: 16421112 Free PMC article.
-
Do the results of pregnancy registries contradict one another?Epilepsy Curr. 2006 May-Jun;6(3):73-5. doi: 10.1111/j.1535-7511.2006.00102.x. Epilepsy Curr. 2006. PMID: 16761065 Free PMC article. No abstract available.
References
-
- Hauser W A, Annegers J F, Rocca W A. Descriptive epidemiology of epilepsy: contributions of population‐based studies from Rochester, Minnesota. Mayo Clin Proc 199671576–586. - PubMed
-
- Dansky L V, Finnell R H. Parental epilepsy, anticonvulsant drugs, and reproductive outcome: epidemiological and experimental findings spanning three decades; 2: human studies. Reprod Toxicol 19915301–335. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical