Chloroquine enhances human CD8+ T cell responses against soluble antigens in vivo
- PMID: 16157687
- PMCID: PMC2212941
- DOI: 10.1084/jem.20051106
Chloroquine enhances human CD8+ T cell responses against soluble antigens in vivo
Abstract
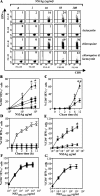
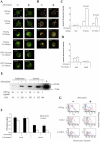
The presentation of exogenous protein antigens in a major histocompatibility complex class I-restricted fashion to CD8+ T cells is called cross-presentation. We demonstrate that cross-presentation of soluble viral antigens (derived from hepatitis C virus [HCV], hepatitis B virus [HBV], or human immunodeficiency virus) to specific CD8+ T cell clones is dramatically improved when antigen-presenting dendritic cells (DCs) are pulsed with the antigen in the presence of chloroquine or ammonium chloride, which reduce acidification of the endocytic system. The export of soluble antigen into the cytosol is considerably higher in chloroquine-treated than in untreated DCs, as detected by confocal microscopy of cultured cells and Western blot analysis comparing endocytic and cytosolic fractions. To pursue our findings in an in vivo setting, we boosted groups of HBV vaccine responder individuals with a further dose of hepatitis B envelope protein vaccine with or without a single dose of chloroquine. Although all individuals showed a boost in antibody titers to HBV, six of nine individuals who were administered chloroquine showed a substantial CD8+ T cell response to HBV antigen, whereas zero of eight without chloroquine lacked a CD8 response. Our results suggest that chloroquine treatment improves CD8 immunity during vaccination.
Figures
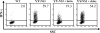



References
-
- Rock, K.L. 1996. A new foreign policy: MHC class I molecules monitor the outside world. Immunol. Today. 17:131–137. - PubMed
-
- Pfeifer, J.D., M.J. Wick, R.L. Roberts, K. Findlay, S.J. Normark, and C.V. Harding. 1993. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 361:359–362. - PubMed
-
- Norbury, C.C., L.J. Hewlett, A.R. Prescott, N. Shastri, and C. Watts. 1995. Class I MHC presentation of exogenous soluble antigen via macropinocytosis in bone marrow macrophages. Immunity. 3:783–791. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

