Syntabulin-mediated anterograde transport of mitochondria along neuronal processes
- PMID: 16157705
- PMCID: PMC1804288
- DOI: 10.1083/jcb.200506042
Syntabulin-mediated anterograde transport of mitochondria along neuronal processes
Abstract
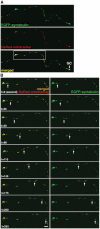
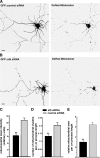
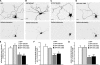
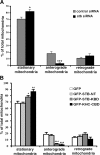
In neurons, proper distribution of mitochondria in axons and at synapses is critical for neurotransmission, synaptic plasticity, and axonal outgrowth. However, mechanisms underlying mitochondrial trafficking throughout the long neuronal processes have remained elusive. Here, we report that syntabulin plays a critical role in mitochondrial trafficking in neurons. Syntabulin is a peripheral membrane-associated protein that targets to mitochondria through its carboxyl-terminal tail. Using real-time imaging in living cultured neurons, we demonstrate that a significant fraction of syntabulin colocalizes and co-migrates with mitochondria along neuronal processes. Knockdown of syntabulin expression with targeted small interfering RNA or interference with the syntabulin-kinesin-1 heavy chain interaction reduces mitochondrial density within axonal processes by impairing anterograde movement of mitochondria. These findings collectively suggest that syntabulin acts as a linker molecule that is capable of attaching mitochondrial organelles to the microtubule-based motor kinesin-1, and in turn, contributes to anterograde trafficking of mitochondria to neuronal processes.
Figures


References
-
- Allen, R.D., J. Metuzals, I. Tasaki, S.T. Brady, and S.P. Gilbert. 1982. Fast axonal transport in squid giant axon. Science. 218:1127–1129. - PubMed
-
- Banker, G.A., and W.M. Cowan. 1979. Further observations on hippocampal neurons in dispersed cell culture. J. Comp. Neurol. 187:469–493. - PubMed
-
- Bordier, C. 1981. Phase separation of integral membrane proteins in Triton X-114 solution. J. Biol. Chem. 256:1604–1607. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

