Stable interaction between alpha5beta1 integrin and Tie2 tyrosine kinase receptor regulates endothelial cell response to Ang-1
- PMID: 16157706
- PMCID: PMC2171441
- DOI: 10.1083/jcb.200507082
Stable interaction between alpha5beta1 integrin and Tie2 tyrosine kinase receptor regulates endothelial cell response to Ang-1
Abstract
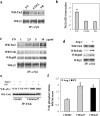
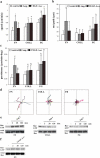
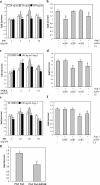
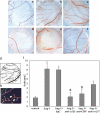
During angiogenic remodeling, Ang-1, the ligand of Tie2 tyrosine kinase, is involved in vessel sprouting and stabilization through unclear effects on nascent capillaries and mural cells. In our study, we hypothesized that the Ang-1/Tie2 system could cross-talk with integrins, and be influenced by the dynamic interactions between extracellular matrix and endothelial cells (ECs). Here, we show that alpha5beta1 specifically sensitizes and modulates Tie2 receptor activation and signaling, allowing EC survival at low concentrations of Ang-1 and inducing persistent EC motility. Tie2 and alpha5beta1 interact constitutively; alpha5beta1 binding to fibronectin increases this association, whereas Ang-1 stimulation recruits p85 and FAK to this complex. Furthermore, we demonstrate that Ang-1 is able to mediate selectively alpha5beta1 outside-in FAK phosphorylation. Thus, Ang-1 triggers signaling pathways through Tie2 and alpha5beta1 receptors that could cross-talk when Tie2/alpha5beta1 interaction occurs in ECs plated on fibronectin. By using blocking antibodies, we consistently found that alpha5beta1, but not alphavbeta3 activation, is essential to Ang-1-dependent angiogenesis in vivo.
Figures


References
-
- Arai, F., A. Hirao, M. Ohmura, H. Sato, S. Matsuoka, K. Takubo, K. Ito, G.Y. Koh, and T. Suda. 2004. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 118:149–161. - PubMed
-
- Armstrong, P.B., and M.T. Armstrong. 2000. Intercellular invasion and the organizational stability of tissues: a role for fibronectin. Biochim. Biophys. Acta. 1470:9–20. - PubMed
-
- Arnaout, M.A., S.L. Goodman, and J.P. Xiong. 2002. Coming to grips with integrin binding to ligands. Curr. Opin. Cell Biol. 14:641–651. - PubMed
-
- Asahara, T., D. Chen, T. Takahashi, K. Fujikawa, M. Kearney, M. Magner, G.D. Yancopoulos, and J.M. Isner. 1998. Tie2 receptor ligands, angiopoietin-1 and angiopoietin-2, modulate VEGF-induced postnatal neovascularization. Circ. Res. 83:233–240. - PubMed
-
- Audero, E., I. Cascone, F. Maniero, L. Napione, M. Arese, L. Lanfrancone, and F. Bussolino. 2004. Adaptor ShcA protein binds tyrosine kinase Tie2 receptor and regulates migration and sprouting but not survival of endothelial cells. J. Biol. Chem. 279:13224–13233. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

