Characterization of a possible amyloidogenic precursor in glutamine-repeat neurodegenerative diseases
- PMID: 16157882
- PMCID: PMC1224618
- DOI: 10.1073/pnas.0502068102
Characterization of a possible amyloidogenic precursor in glutamine-repeat neurodegenerative diseases
Abstract
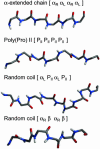
Several neurodegenerative diseases are linked to expanded repeats of glutamine residues, which lead to the formation of amyloid fibrils and neuronal death. The length of the repeats correlates with the onset of Huntington's disease, such that healthy individuals have <38 residues and individuals with >38 repeats exhibit symptoms. Because it is difficult to obtain atomic-resolution structural information for poly(l-glutamine) (polyQ) in aqueous solution experimentally, we performed molecular dynamics simulations to investigate the conformational behavior of this homopolymer. In simulations of 20-, 40-, and 80-mer polyQ, we observed the formation of the "alpha-extended chain" conformation, which is characterized by alternating residues in the alpha(L) and alpha(R) conformations to yield a sheet. The structural transition from disordered random-coil conformations to the alpha-extended chain conformation exhibits modest length and temperature dependence, in agreement with the experimental observation that aggregation depends on length and temperature. We propose that fibril formation in polyQ may occur through an alpha-sheet structure, which was proposed by Pauling and Corey. Also, we propose an atomic-resolution model of how the inhibitory peptide QBP1 (polyQ-binding peptide 1) may bind to polyQ in an alpha-extended chain conformation to inhibit fibril formation.
Figures

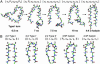

References
-
- Perutz, M. F. (1999) Trends Biochem. Sci. 24, 58-63. - PubMed
-
- Davies, S. W., Turmaine, M., Cozens, B. A., Difigla, M., Sharp, A. H., Ross, C. A., Scherzinger, E., Wanker, E. E., Mangiarini, L. & Bates, G. P. (1997) Cell 90, 537-548. - PubMed
-
- Perutz, M. F. (1997) Curr. Opin. Struct. Biol. 6, 848-858. - PubMed
-
- Gusella, J. F. & MacDonald, M. E. (2002) Nat. Rev. Neurosci. 1, 109-115. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

