The biological effects of syngeneic and allogeneic cytokine-expressing prophylactic whole cell vaccines and the influence of irradiation in a murine melanoma model
- PMID: 16158275
- PMCID: PMC11030598
- DOI: 10.1007/s00262-005-0061-2
The biological effects of syngeneic and allogeneic cytokine-expressing prophylactic whole cell vaccines and the influence of irradiation in a murine melanoma model
Abstract
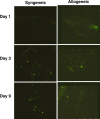
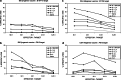
Allogeneic whole tumour cell vaccines are inherently practical compared with autologous vaccines. Cell lines are derived from allogeneic tumour, grown in bulk and then administered as a vaccine to the patient, following irradiation, which not only prevents any replication but also enhances antigen presentation. Protection is believed to occur through the presentation of antigens shared between the syngeneic and allogeneic tumours. Although cytokine-transfected tumour whole cell vaccines have been used clinically, little data is available comparing the effects of immunomodulatory cytokine-transfection directly on the same cells when used as both an allogeneic and autologous vaccine. To address this, weakly immunogenic B16-F10 (H-2b) murine melanoma was transfected to secrete either GM-CSF, IL-4 or IL-7. Prophylactic vaccination of both syngeneic C57/BL6 (H-2b) (B6) and allogeneic C3H/Hej (H-2k) (C3H) mice showed the effects of transfected cytokine varied between models. Both GM-CSF and IL-7 significantly (P<0.05) increased the levels of protection within syngeneic B6 mice, but had a diminished effect (P>0.05) within C3H allogeneic mice. Allogeneic B16-F10 cells and syngeneic K1735 cells generated CTL against K1735 suggesting cross-reactive immunity. Using cells labeled with fluorescent dye we demonstrate that irradiated vaccines, of either syngeneic or allogeneic origin, appear to generate potent immune responses and fragments of either vaccine remain at the injection site for up to 9 days. This study shows that protection can be enhanced in vivo by using transfected cytokine, but suggests that irradiated whole cell vaccines, of either tissue-type, are rapidly processed. This leads to the conclusion that the cytokine effects are transient and thus transfection with cytokine may be of limited long-term use in situ.
Figures




Similar articles
-
Efficacy of cytokine gene transfection may differ for autologous and allogeneic tumour cell vaccines.Immunology. 2001 Feb;102(2):190-8. doi: 10.1046/j.1365-2567.2001.01176.x. Immunology. 2001. PMID: 11260324 Free PMC article.
-
Anti-tumour activity against B16-F10 melanoma with a GM-CSF secreting allogeneic tumour cell vaccine.Gene Ther. 1999 Aug;6(8):1475-81. doi: 10.1038/sj.gt.3300961. Gene Ther. 1999. PMID: 10467372
-
A novel murine model of allogeneic vaccination against prostate cancer.Cancer Immunol Immunother. 2008 Apr;57(4):453-65. doi: 10.1007/s00262-007-0384-2. Epub 2007 Sep 6. Cancer Immunol Immunother. 2008. PMID: 17805533 Free PMC article.
-
Reduced efficacy of allogeneic versus syngeneic fibroblasts modified to secrete cytokines as a tumor vaccine adjuvant.Cancer Res. 1997 Aug 1;57(15):3230-7. Cancer Res. 1997. PMID: 9242454
-
Pulsing of dendritic cells with cell lysates from either B16 melanoma or MCA-106 fibrosarcoma yields equally effective vaccines against B16 tumors in mice.J Surg Oncol. 1998 Jun;68(2):79-91. doi: 10.1002/(sici)1096-9098(199806)68:2<79::aid-jso3>3.0.co;2-h. J Surg Oncol. 1998. PMID: 9624036 Review.
Cited by
-
Therapeutic efficacy of PD-L1 blockade in a breast cancer model is enhanced by cellular vaccines expressing B7-1 and glycolipid-anchored IL-12.Hum Vaccin Immunother. 2016;12(2):421-30. doi: 10.1080/21645515.2015.1076953. Hum Vaccin Immunother. 2016. PMID: 26308597 Free PMC article.
-
Design and characterization of the tumor vaccine MGN1601, allogeneic fourfold gene-modified vaccine cells combined with a TLR-9 agonist.Mol Ther Oncolytics. 2016 Feb 10;3:15023. doi: 10.1038/mto.2015.23. eCollection 2016. Mol Ther Oncolytics. 2016. PMID: 27119114 Free PMC article.
-
Vaccination against prostate cancer using a live tissue factor deficient cell line in Lobund-Wistar rats.Cancer Immunol Immunother. 2007 May;56(5):725-30. doi: 10.1007/s00262-006-0223-x. Epub 2006 Sep 5. Cancer Immunol Immunother. 2007. PMID: 16953436 Free PMC article.
References
-
- Arienti F, Belli F, Napolitano F, Sule-Suso J, Mazzocchi A, Gallino GF, Cattelan A, Santantonio C, Rivoltini L, Melani C, Colombo MP, Cascinelli N, Maio M, Parmiani G, Sanantonio C. Vaccination of melanoma patients with interleukin 4 gene-transduced allogeneic melanoma cells. Hum Gene Ther. 1999;10:2907. doi: 10.1089/10430349950016320. - DOI - PubMed
-
- Berd D, Maguire HC, Jr, McCue P, Mastrangelo MJ. Treatment of metastatic melanoma with an autologous tumor-cell vaccine: clinical and immunologic results in 64 patients. J Clin Oncol. 1990;8:1858. - PubMed
-
- Chong H, Todryk S, Hutchinson G, Hart IR, Vile RG. Tumour cell expression of B7 costimulatory molecules and interleukin-12 or granulocyte-macrophage colony-stimulating factor induces a local antitumour response and may generate systemic protective immunity. Gene Ther. 1998;5:223. doi: 10.1038/sj.gt.3300584. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

