Anthracyclines, proteasome activity and multi-drug-resistance
- PMID: 16159384
- PMCID: PMC1242219
- DOI: 10.1186/1471-2407-5-114
Anthracyclines, proteasome activity and multi-drug-resistance
Abstract
Background: P-glycoprotein is responsible for the ATP-dependent export of certain structurally unrelated compounds including many chemotherapeutic drugs. Amplification of P-glycoprotein activity can result in multi-drug resistance and is a common cause of chemotherapy treatment failure. Therefore, there is an ongoing search for inhibitors of P-glycoprotein. Observations that cyclosporin A, and certain other substances, inhibit both the proteasome and P-glycoprotein led us to investigate whether anthracyclines, well known substrates of P-gp, also inhibit the function of the proteasome.
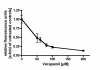
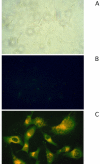
Methods: Proteasome function was measured in cell lysates from ECV304 cells incubated with different doses of verapamil, doxorubicin, daunorubicin, idarubicin, epirubicin, topotecan, mitomycin C, and gemcitabine using a fluorogenic peptide assay. Proteasome function in living cells was monitored using ECV304 cells stably transfected with the gene for an ubiquitin/green fluorescent protein fusion protein. The ability of the proteasome inhibitor MG-132 to affect P-glycoprotein function was monitored by fluorescence due to accumulation of daunorubicin in P-glycoprotein overexpressing KB 8-5 cells.
Results: Verapamil, daunorubicin, doxorubicin, idarubicin, and epirubicin inhibited 26S chymotrypsin-like function in ECV304 extracts in a dose-dependent fashion. With the exception of daunorubicin, 20S proteasome function was also suppressed. The proteasome inhibitor MG-132 caused a dose-dependent accumulation of daunorubicin in KB 8-5 cells that overexpress P-glycoprotein, suggesting that it blocked P-glycoprotein function.
Conclusion: Our data indicate that anthracyclines inhibit the 26S proteasome as well as P-glycoprotein. Use of inhibitors of either pathway in cancer therapy should take this into consideration and perhaps use it to advantage, for example during chemosensitization by proteasome inhibitors.
Figures
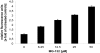
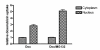
References
-
- Harding CV, France J, Song R, Farah JM, Chatterjee S, Iqbal M, Siman R. Novel dipeptide aldehydes are proteasome inhibitors and block the MHC-I antigen-processing pathway. J Immunol. 1995;155:1767–1775. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

