Ethanol induces cell-cycle activity and reduces stem cell diversity to alter both regenerative capacity and differentiation potential of cerebral cortical neuroepithelial precursors
- PMID: 16159388
- PMCID: PMC1249578
- DOI: 10.1186/1471-2202-6-59
Ethanol induces cell-cycle activity and reduces stem cell diversity to alter both regenerative capacity and differentiation potential of cerebral cortical neuroepithelial precursors
Abstract
Background: The fetal cortical neuroepithelium is a mosaic of distinct progenitor populations that elaborate diverse cellular fates. Ethanol induces apoptosis and interferes with the survival of differentiating neurons. However, we know little about ethanol's effects on neuronal progenitors. We therefore exposed neurosphere cultures from fetal rat cerebral cortex, to varying ethanol concentrations, to examine the impact of ethanol on stem cell fate.
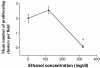
Results: Ethanol promoted cell cycle progression, increased neurosphere number and increased diversity in neurosphere size, without inducing apoptosis. Unlike controls, dissociated cortical progenitors exposed to ethanol exhibited morphological evidence for asymmetric cell division, and cells derived from ethanol pre-treated neurospheres exhibited decreased proliferation capacity. Ethanol significantly reduced the numbers of cells expressing the stem cell markers CD117, CD133, Sca-1 and ABCG2, without decreasing nestin expression. Furthermore, ethanol-induced neurosphere proliferation was not accompanied by a commensurate increase in telomerase activity. Finally, cells derived from ethanol-pretreated neurospheres exhibited decreased differentiation in response to retinoic acid.
Conclusion: The reduction in stem cell number along with a transient ethanol-driven increase in cell proliferation, suggests that ethanol promotes stem to blast cell maturation, ultimately depleting the reserve proliferation capacity of neuroepithelial cells. However, the lack of a concomitant change in telomerase activity suggests that neuroepithelial maturation is accompanied by an increased potential for genomic instability. Finally, the cellular phenotype that emerges from ethanol pre-treated, stem cell depleted neurospheres is refractory to additional differentiation stimuli, suggesting that ethanol exposure ablates or delays subsequent neuronal differentiation.
Figures








References
-
- Coles CD, Platzman KA, Raskind-Hood CL, Brown RT, Falek A, Smith IE. A comparison of children affected by prenatal alcohol exposure and attention deficit, hyperactivity disorder. Alcohol Clin Exp Res. 1997;21 :150–161. - PubMed
-
- Mattson SN, Riley EP, Delis DC, Stern C, Jones KL. Verbal learning and memory in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 1996;20 :810–816. - PubMed
-
- Mattson SN, Goodman AM, Caine C, Delis DC, Riley EP. Executive functioning in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 1999;23 :1808–1815. - PubMed
-
- Roebuck TM, Simmons RW, Mattson SN, Riley EP. Prenatal exposure to alcohol affects the ability to maintain postural balance. Alcohol Clin Exp Res. 1998;22 :252–258. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

