Atorvastatin inhibits hypercholesterolemia-induced calcification in the aortic valves via the Lrp5 receptor pathway
- PMID: 16159822
- PMCID: PMC3951868
- DOI: 10.1161/01.CIRCULATIONAHA.104.524306
Atorvastatin inhibits hypercholesterolemia-induced calcification in the aortic valves via the Lrp5 receptor pathway
Erratum in
- Circulation. 2010 Jun 29;121(25):e458
Abstract
Background: Calcific aortic valve disease is the most common indication for surgical valve replacement in the United States. The cellular mechanisms of valve calcification are not well understood. We have previously shown that cellular proliferation and osteoblastogenesis are important in the development of valvular heart disease. Lrp5, a known low-density receptor-related protein, plays an essential role in cellular proliferation and osteoblastogenesis via the beta-catenin signaling pathway. We hypothesize that Lrp5 also plays a role in aortic valve (AV) calcification in experimental hypercholesterolemia.
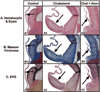
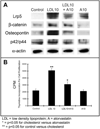
Methods and results: We examined the effects of cholesterol and atorvastatin in Watanabe rabbits (n=54). Group I (n=18) received a normal diet, group II (n=18) a 0.25% cholesterol diet, and group III (n=18) a 0.25% (w/w) cholesterol diet with atorvastatin for the development of calcification. The AVs were examined for cellular proliferation, Lrp5/beta-catenin, and bone matrix markers. Bone formation was assessed by micro-computed tomography, calcein injection, and osteopontin expression. Low-density lipoprotein with and without atorvastatin was also tested in AV myofibroblasts for cellular proliferation and regulation of the Lrp5/beta-catenin pathway. Our results demonstrate that the cholesterol diet induced complex bone formations in the calcified AVs with an increase in the Lrp5 receptors, osteopontin, and p42/44 expression. Atorvastatin reduced bone formation, cellular proliferation, and Lrp5/beta-catenin protein levels in the AVs. In vitro analysis confirmed the Lrp5/beta-catenin expression in myofibroblast cell proliferation.
Conclusions: Hypercholesterolemic AV calcification is attenuated by atorvastatin and is mediated in part by the Lrp5/beta-catenin pathway. This developmental pathway may be important in the signaling pathway of this disease.
Figures


References
-
- Bonow RO, Carabello B, de Leon AC, Edmunds LH, Jr, Fedderly BJ, Freed MD, Gaasch WH, McKay CR, Nishimura RA, O’Gara PT, O’Rourke RA, Rahimtoola SH, Ritchie JL, Cheitlin MD, Eagle KA, Gardner TJ, Garson A, Jr, Gibbons RJ, Russell RO, Ryan TJ, Smith SC., Jr ACC/AHA Guidelines for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association task force on Practice Guidelines (Committee on Management of Patients With Valvular Heart Disease) J Heart Valve Dis. 1998;7:672–707. - PubMed
-
- STS TECOM web site. [Accessed March 3, 2005]; Available at: http://www.sts.com.
-
- O’Brien KD, Kuusisto J, Reichenbach DD, Ferguson M, Giachelli C, Alpers CE, Otto CM. Osteopontin is expressed in human aortic valvular lesions. Circulation. 1995;92:2163–2168. - PubMed
-
- Mohler ER, 3rd, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation. 2001;103:1522–1528. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

