Immunoregulation of dendritic cells
- PMID: 16160071
- PMCID: PMC1237158
- DOI: 10.3121/cmr.3.3.166
Immunoregulation of dendritic cells
Abstract
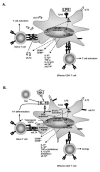
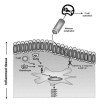
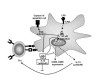
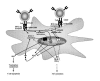
The paradigm of tolerogenic/immature versus inflammatory/mature dendritic cells has dominated the recent literature regarding the role of these antigen-presenting cells in mediating immune homeostasis or self-tolerance and response to pathogens, respectively. This issue is further complicated by the identification of distinct subtypes of dendritic cells that exhibit different antigen-presenting cell effector functions. The discovery of pathogen-associated molecular patterns and toll-like receptors provides the mechanistic basis for dendritic cell recognition of specific pathogens and induction of appropriate innate and adaptive immune responses. Only recently has insight been gained into how dendritic cells contribute to establishing and/or maintaining immunological tolerance to self. Soluble and cellular mediators have been reported to effectively regulate the function of dendritic cells by inducing several outcomes ranging from non-inflammatory dendritic cells that lack the ability to induce T lymphocyte activation to dendritic cells that actively suppress T lymphocyte responses. A thorough discussion of these stimuli and their outcomes is essential to understanding the potential for modulating dendritic cell function in the treatment of inflammatory disease conditions.
Figures
References
-
- Alaniz RC, Sandall S, Thomas EK, Wilson CB. Increased dendritic cell numbers impair protective immunity to intracellular bacteria despite augmenting antigen-specific CD8+ T lymphocyte responses. J Immunol 2004;172:3725–3735. - PubMed
-
- Maldonado-Lopez R, Moser M. Dendritic cell subsets and the regulation of Th1/Th2 responses. Semin Immunol 2001;13:275–282. - PubMed
-
- Eisenbarth SC, Piggott DA, Bottomly K. The master regulators of allergic inflammation: dendritic cells in Th2 sensitization. Curr Opin Immunol 2003;15:620–626. - PubMed
-
- Smith CM, Wilson NS, Waithman J, Villadangos JA, Carbone FR, Heath WR, Belz GT. Cognate CD4(+) T cell licensing of dendritic cells in CD8(+) T cell immunity. Nat Immunol 2004;5:1143–1148. - PubMed
-
- Gerloni M, Lo D, Zanetti M. DNA immunization in relB-deficient mice discloses a role for dendritic cells in IgM—>IgG1 switch in vivo. Eur J Immunol 1998;28:516–524. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
