Regulation of human immunodeficiency virus type 1 envelope glycoprotein fusion by a membrane-interactive domain in the gp41 cytoplasmic tail
- PMID: 16160149
- PMCID: PMC1211532
- DOI: 10.1128/JVI.79.19.12231-12241.2005
Regulation of human immunodeficiency virus type 1 envelope glycoprotein fusion by a membrane-interactive domain in the gp41 cytoplasmic tail
Abstract
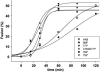
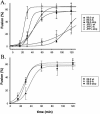
Truncation of the human immunodeficiency virus (HIV) or simian immunodeficiency virus (SIV) gp41 cytoplasmic tail (CT) can modulate the fusogenicity of the envelope glycoprotein (Env) on infected cells and virions. However, the CT domains involved and the underlying mechanism responsible for this "inside-out" regulation of Env function are unknown. HIV and SIV CTs are remarkably long and contain amphipathic alpha-helical domains (LLP1, LLP2, and LLP3) that likely interact with cellular membranes. Using a cell-cell fusion assay and a panel of HIV Envs with stop codons at various positions in the CT, we show that truncations of gp41 proximal to the most N-terminal alpha helix, LLP2, increase fusion efficiency and expose CD4-induced epitopes in the Env ectodomain. These effects were not seen with a truncation distal to this domain and before LLP1. Using a dye transfer assay to quantitate fusion kinetics, we found that these truncations produced a two- to fourfold increase in the rate of fusion. These results were observed for X4-, R5-, and dual-tropic Envs on CXCR4- and CCR5-expressing target cells and could not be explained by differences in Env surface expression. These findings suggest that distal to the membrane-spanning domain, an interaction of the gp41 LLP2 domain with the cell membrane restricts Env fusogenicity during Env processing. As with murine leukemia viruses, where cleavage of a membrane-interactive R peptide at the C terminus is required for Env to become fusogenic, this restriction of Env function may serve to protect virus-producing cells from the membrane-disruptive effects of the Env ectodomain.
Figures




Similar articles
-
Role of envelope processing and gp41 membrane spanning domain in the formation of human immunodeficiency virus type 1 (HIV-1) fusion-competent envelope glycoprotein complex.Virus Res. 2007 Mar;124(1-2):103-12. doi: 10.1016/j.virusres.2006.10.009. Epub 2006 Nov 28. Virus Res. 2007. PMID: 17129629
-
Role of the fusion peptide and membrane-proximal domain in HIV-1 envelope glycoprotein-mediated membrane fusion.Biochemistry. 2003 Dec 9;42(48):14150-8. doi: 10.1021/bi035154g. Biochemistry. 2003. PMID: 14640682
-
Surface exposure of the HIV-1 env cytoplasmic tail LLP2 domain during the membrane fusion process: interaction with gp41 fusion core.J Biol Chem. 2008 Jun 13;283(24):16723-31. doi: 10.1074/jbc.M801083200. Epub 2008 Apr 11. J Biol Chem. 2008. PMID: 18408000 Free PMC article.
-
HIV-1 envelope glycoprotein biosynthesis, trafficking, and incorporation.J Mol Biol. 2011 Jul 22;410(4):582-608. doi: 10.1016/j.jmb.2011.04.042. J Mol Biol. 2011. PMID: 21762802 Free PMC article. Review.
-
Molecular Mechanism of HIV-1 Entry.Trends Microbiol. 2019 Oct;27(10):878-891. doi: 10.1016/j.tim.2019.06.002. Epub 2019 Jun 28. Trends Microbiol. 2019. PMID: 31262533 Free PMC article. Review.
Cited by
-
Laser capture microdissection assessment of virus compartmentalization in the central nervous systems of macaques infected with neurovirulent simian immunodeficiency virus.J Virol. 2013 Aug;87(16):8896-908. doi: 10.1128/JVI.00874-13. Epub 2013 May 29. J Virol. 2013. PMID: 23720733 Free PMC article.
-
Clustering and mobility of HIV-1 Env at viral assembly sites predict its propensity to induce cell-cell fusion.J Virol. 2013 Jul;87(13):7516-25. doi: 10.1128/JVI.00790-13. Epub 2013 May 1. J Virol. 2013. PMID: 23637402 Free PMC article.
-
Maturation-dependent human immunodeficiency virus type 1 particle fusion requires a carboxyl-terminal region of the gp41 cytoplasmic tail.J Virol. 2007 Sep;81(18):9999-10008. doi: 10.1128/JVI.00592-07. Epub 2007 Jul 3. J Virol. 2007. PMID: 17609279 Free PMC article.
-
Conserved arginine residue in the membrane-spanning domain of HIV-1 gp41 is required for efficient membrane fusion.Protein Cell. 2011 May;2(5):369-76. doi: 10.1007/s13238-011-1051-0. Epub 2011 Jun 12. Protein Cell. 2011. PMID: 21667332 Free PMC article.
-
Enhanced fusion pore expansion mediated by the trans-acting Endodomain of the reovirus FAST proteins.PLoS Pathog. 2009 Mar;5(3):e1000331. doi: 10.1371/journal.ppat.1000331. Epub 2009 Mar 6. PLoS Pathog. 2009. PMID: 19266079 Free PMC article.
References
-
- Andreassen, H., H. Bohr, J. Bohr, S. Brunak, T. Bugge, R. M. Cotterill, C. Jacobsen, P. Kusk, B. Lautrup, S. B. Petersen, et al. 1990. Analysis of the secondary structure of the human immunodeficiency virus (HIV) proteins p17, gp120, and gp41 by computer modeling based on neural network methods. J. Acquir. Immune. Defic. Syndr. 3:615-622. - PubMed
-
- Babcock, G. J., M. Farzan, and J. Sodroski. 2003. Ligand-independent dimerization of CXCR4, a principal HIV-1 coreceptor. J. Biol. Chem. 278:3378-3385. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

