Inactivation of retroviruses with preservation of structural integrity by targeting the hydrophobic domain of the viral envelope
- PMID: 16160166
- PMCID: PMC1211527
- DOI: 10.1128/JVI.79.19.12394-12400.2005
Inactivation of retroviruses with preservation of structural integrity by targeting the hydrophobic domain of the viral envelope
Abstract
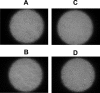
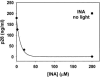
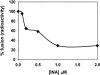
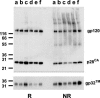
We describe a new approach for the preparation of inactivated retroviruses for vaccine application. The lipid domain of the viral envelope was selectively targeted to inactivate proteins and lipids therein and block fusion of the virus with the target cell membrane. In this way, complete elimination of the infectivity of human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) could be achieved with preservation of antigenic determinants on the surface of the viral envelope. Inactivation was accomplished by modification of proteins and lipids in the viral envelope using the hydrophobic photoinduced alkylating probe 1,5 iodonaphthylazide (INA). Treatment of HIV and SIV isolates with INA plus light completely blocked fusion of the viral envelope and abolished infectivity. The inactivated virus remained structurally unchanged, with no detectable loss of viral proteins. Modifications to envelope and nucleocapsid proteins were detected by changes in their elution pattern on reverse-phase high-performance liquid chromatography. These modifications had no effect on primary and secondary structure epitopes as determined by monoclonal antibodies. Likewise, the inactivated HIV reacted as well as the live virus with the conformation-sensitive and broadly neutralizing anti-HIV type 1 monoclonal antibodies 2G12, b12, and 4E10. Targeting the lipid domain of biological membranes with hydrophobic alkylating compounds could be used as a general approach for inactivation of enveloped viruses and other pathogenic microorganisms for vaccine application.
Figures




Similar articles
-
Characterization of the effects of aryl-azido compounds and UVA irradiation on the viral proteins and infectivity of human immunodeficiency virus type 1.Photochem Photobiol. 2010 Sep-Oct;86(5):1099-108. doi: 10.1111/j.1751-1097.2010.00780.x. Photochem Photobiol. 2010. PMID: 20630026 Free PMC article.
-
Hydrophobic inactivation of influenza viruses confers preservation of viral structure with enhanced immunogenicity.J Virol. 2008 May;82(9):4612-9. doi: 10.1128/JVI.02233-07. Epub 2008 Feb 27. J Virol. 2008. PMID: 18305038 Free PMC article.
-
Chemical inactivation of retroviral infectivity by targeting nucleocapsid protein zinc fingers: a candidate SIV vaccine.AIDS Res Hum Retroviruses. 1998 Oct;14 Suppl 3:S311-9. AIDS Res Hum Retroviruses. 1998. PMID: 9814959
-
Sites, mechanism of action and lack of reversibility of primate lentivirus inactivation by preferential covalent modification of virion internal proteins.Curr Mol Med. 2003 May;3(3):265-72. doi: 10.2174/1566524033479889. Curr Mol Med. 2003. PMID: 12699362 Review.
-
Simian and feline immunodeficiency viruses: animal lentivirus models for evaluation of AIDS vaccines and antiviral agents.Antiviral Res. 1991 May;15(4):267-86. doi: 10.1016/0166-3542(91)90009-g. Antiviral Res. 1991. PMID: 1659310 Review.
Cited by
-
Characterization of the effects of aryl-azido compounds and UVA irradiation on the viral proteins and infectivity of human immunodeficiency virus type 1.Photochem Photobiol. 2010 Sep-Oct;86(5):1099-108. doi: 10.1111/j.1751-1097.2010.00780.x. Photochem Photobiol. 2010. PMID: 20630026 Free PMC article.
-
Dengue virus photo-inactivated in presence of 1,5-iodonaphthylazide (INA) or AMT, a psoralen compound (4'-aminomethyl-trioxsalen) is highly immunogenic in mice.Hum Vaccin Immunother. 2013 Nov;9(11):2336-41. doi: 10.4161/hv.25602. Epub 2013 Jul 8. Hum Vaccin Immunother. 2013. PMID: 23835446 Free PMC article.
-
Mycoplasma gallisepticum inactivated by targeting the hydrophobic domain of the membrane preserves surface lipoproteins and induces a strong immune response.PLoS One. 2015 Mar 17;10(3):e0120462. doi: 10.1371/journal.pone.0120462. eCollection 2015. PLoS One. 2015. PMID: 25781939 Free PMC article.
-
Inactivation of avian influenza viruses by hydrostatic pressure as a potential vaccine development approach.Access Microbiol. 2021 Apr 13;3(4):000220. doi: 10.1099/acmi.0.000220. eCollection 2021. Access Microbiol. 2021. PMID: 34151171 Free PMC article.
-
Hydrophobic inactivation of influenza viruses confers preservation of viral structure with enhanced immunogenicity.J Virol. 2008 May;82(9):4612-9. doi: 10.1128/JVI.02233-07. Epub 2008 Feb 27. J Virol. 2008. PMID: 18305038 Free PMC article.
References
-
- Arthur, O. L., J. W. Bess, Jr., E. N. Chertova, J. L. Rossio, M. T. Esser, R. E. Benveniste, L. E. Henderson, and J. D. Lifson. 1998. Chemical inactivation of retroviral infectivity by targeting nucleocapsid protein zinc fingers: a candidate SIV vaccine. AIDS Res. Hum. Retrovir. 14:S311-S319. - PubMed
-
- Baba, W. T., V. Liska, R. H. Lehman, J. Vlasak, W. Xu, S. Ayehunie, L. C. Cavacini, M. R. Posner, H. Kattinger, G. Stiegler, B. R. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, Y. Lu, j. E. Wright, T. C. Chou, and R. M. Ruprecht. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6:200-206. - PubMed
-
- Benade, E. L., Y. Shumaker, X. X. Chen, and Y. R. Dodd. 1994. Inactivation of free and cell-associated human immunodeficiency virus in platelet suspensions by aminomethyltrimethylpsoralen and ultraviolet light. Transfusion 34:680-684. - PubMed
-
- Bercovici, T., and C. Gitler. 1978. 5-[125I]iodonaphthyl azide, a reagent to determine the penetration of proteins into the lipid bilayer of biological membranes. Biochemistry 17:1484-1489. - PubMed
-
- Blanco, J., J. Barretina, B. Clotet, and A. J. Este. 2004. R5 HIV gp120-mediated cellular contacts induce the death of single CCR5-expressing CD4 T cells by a gp41-dependent mechanism. J. Leukoc. Biol. 76:804-881. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

