Side effects during chemotherapy predict tumour response in advanced colorectal cancer
- PMID: 16160693
- PMCID: PMC2361647
- DOI: 10.1038/sj.bjc.6602783
Side effects during chemotherapy predict tumour response in advanced colorectal cancer
Abstract
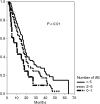
To investigate whether a relationship between chemotherapy-associated adverse events and treatment efficacy exists, we have analysed the toxicity, objective response and survival data of 303 patients with advanced colorectal cancer. Patients were divided into two groups: the first with beneficial effect (I, n = 245), and the second with progressive disease (II, n = 58). Differences in terms of incidence rates, type and severity of ad verse events were analysed with univariate and multivariate models. The median number of side effects in group I was 6 vs 4 in group II (OR=1.342; P= 0.0001). An inverse correlation between disease control and treatment tolerance was confirmed when side effects were analysed according to severity and type of treatment-associated toxicities (haematological: P = 0.0005 vs nonhaematological P = 0.0001). When median survival was analysed according to the number of adverse events, it was 10 (95% CI, 3-7), 16 (14-18), and 18 (16-20) months in case of 0-1, 2-5, and > or =6 adverse events, respectively (P = 0.01). In conclusion, the results of this analysis suggest that occurrence of side effects during chemotherapy in advanced colorectal cancer is an independent and reliable prognostic indicator for response and survival.
Figures
References
-
- Ansfield FJ (1975) A randomized phase III study of four dosage regimens of 5-fluorouracil. Proc Am Assoc Cancer Res 16: 224 (abstract)
-
- Blum J, Jones S, Buzdar A (2001) Capecitabine (Xeloda) in 162 patients with paclitaxel-pretreated MBC: updated results and analyses of dose modification (abstract 693). Eur J Cancer 37(Suppl 6): S190 (abstract)
-
- Cascinu S, Del Ferro E, Catalano G (1996) Toxicity and therapeutic response to chemotherapy in patients aged 70 years or older with advanced cancer. Am J Clin Oncol 19: 371–374 - PubMed
-
- Cassidy J, Twelves C, Van Cutsem E, Hoff P, Bajetta E, Boyer M, Bugat R, Burger U, Garin A, Graeven U, McKendric J, Maroun J, Marshall J, Osterwalder B, Perez-Manga G, Rosso R, Rougier P, Schilsky RL (2002) First-line oral capecitabine therapy in metastatic colorectal cancer: a favorable safety profile compared with iv 5-fluorouracil/leucovorin. Ann Oncol 13: 566–575 - PubMed
-
- Chiara S, Nobile MT, Vincenti M, Lionetto R, Gozza A, Barzacchi MC, Sanguineti O, Repetto L, Rosso R (1998) Advanced colorectal cancer in the elderly: results of consecutive trials with 5-fluorouracil-based chemotherapy. Cancer Chemother Pharmacol 42: 336–340 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical