Marked differences in human melanoma antigen-specific T cell responsiveness after vaccination using a functional microarray
- PMID: 16162034
- PMCID: PMC1216330
- DOI: 10.1371/journal.pmed.0020265
Marked differences in human melanoma antigen-specific T cell responsiveness after vaccination using a functional microarray
Abstract
Background: In contrast to many animal model studies, immunotherapeutic trials in humans suffering from cancer invariably result in a broad range of outcomes, from long-lasting remissions to no discernable effect.
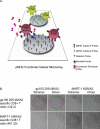
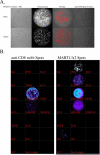
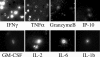
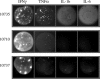
Methods and findings: In order to study the T cell responses in patients undergoing a melanoma-associated peptide vaccine trial, we have developed a high-throughput method using arrays of peptide-major histocompatibility complexes (pMHC) together with antibodies against secreted factors. T cells were specifically immobilized and activated by binding to particular pMHCs. The antibodies, spotted together with the pMHC, specifically capture cytokines secreted by the T cells. This technique allows rapid, simultaneous isolation and multiparametric functional characterization of antigen-specific T cells present in clinical samples. Analysis of CD8+ lymphocytes from ten melanoma patients after peptide vaccination revealed a diverse set of patient- and antigen-specific profiles of cytokine secretion, indicating surprising differences in their responsiveness. Four out of four patients who showed moderate or greater secretion of both interferon-gamma (IFNgamma) and tumor necrosis factor-alpha (TNFalpha) in response to a gp100 antigen remained free of melanoma recurrence, whereas only two of six patients who showed discordant secretion of IFNgamma and TNFalpha did so.
Conclusion: Such multiparametric analysis of T cell antigen specificity and function provides a valuable tool with which to dissect the molecular underpinnings of immune responsiveness and how this information correlates with clinical outcome.
Conflict of interest statement
Figures



Comment in
-
Cancer vaccines: the next generation of tools to monitor the anticancer immune response.PLoS Med. 2005 Oct;2(10):e339. doi: 10.1371/journal.pmed.0020339. Epub 2005 Oct 25. PLoS Med. 2005. PMID: 16231977 Free PMC article. Review.
References
-
- Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380–384. - PubMed
-
- van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–1647. - PubMed
-
- Lee PP, Yee C, Savage PA, Fong L, Brockstedt D, et al. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med. 1999;5:677–685. - PubMed
-
- Talebi T, Weber JS. Peptide vaccine trials for melanoma: Preclinical background and clinical results. Semin Cancer Biol. 2003;13:431–438. - PubMed
-
- Kessels HW, Wolkers MC, Schumacher TN. Adoptive transfer of T-cell immunity. Trends Immunol. 2002;23:264–269. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

