Different approaches for assaying melanosome transfer
- PMID: 16162177
- PMCID: PMC1360235
- DOI: 10.1111/j.1600-0749.2005.00263.x
Different approaches for assaying melanosome transfer
Abstract
Many approaches have been tried to establish assays for melanosome transfer to keratinocytes. In this report, we describe and summarize various novel attempts to label melanosomes in search of a reliable, specific, reproducible and quantitative assay system. We tried to fluorescently label melanosomes by transfection of GFP-labeled melanosomal proteins and by incubation of melanocytes with fluorescent melanin intermediates or homologues. In most cases a weak cytoplasmic fluorescence was perceived, which was probably because of incorrect sorting or deficient incorporation of the fluorescent protein and different localization. We were able to label melanosomes via incorporation of 14C-thiouracil into melanin. Consequently, we tried to develop an assay to separate keratinocytes with transferred radioactivity from melanocytes after co-culture. Differential trypsinization and different magnetic bead separation techniques were tested with unsatisfactory results. An attempt was also made to incorporate fluorescent thiouracil, since this would allow cells to be separated by FACS. In conclusion, different methods to measure pigment transfer between donor melanocytes and acceptor keratinocytes were thoroughly examined. This information could give other researchers a head start in the search for a melanosome transfer assay with said qualities to better understand pigment transfer.
Figures
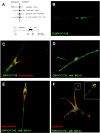
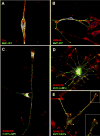
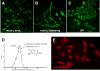


References
-
- Adams SR, Campbell RE, Gross LA, Martin BR, Walkup GK, Yao Y, Llopis J, Tsien RY. New biarsenical ligands and tetracysteine motifs for protein labeling in vitro and in vivo: synthesis and biological applications. J Am Chem Soc. 2002;124:6063–6076. - PubMed
-
- Ancans J, Thody AJ. Activation of melanogenesis by vacuolar type H(+)-ATPase inhibitors in amelanotic, tyrosinase positive human and mouse melanoma cells. FEBS Lett. 2000;478:57–60. - PubMed
-
- Babiarz-Magee L, Chen N, Seiberg M, Lin CB. The expression and activation of protease-activated receptor-2 correlate with skin color. Pigment Cell Res. 2004;17:241–251. - PubMed
-
- Bahadoran P, Busca R, Chiaverini C, Westbroek W, Lambert J, Bille K, Valony G, Fukuda M, Naeyaert JM, Ortonne JP, Ballotti R. Characterization of the molecular defects in Rab27a, caused by RAB27A missense mutations found in patients with Griscelli syndrome. J Biol Chem. 2003;278:11386–11392. - PubMed
-
- Basrur V, Yang F, Kushimoto T, Higashimoto Y, Yasumoto K, Valencia J, Muller J, Vieira WD, Watabe H, Shabanowitz J, et al. Proteomic analysis of early melanosomes: identification of novel melanosomal proteins. J Proteome Res 2003;2:69–79. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

