Both antigen optimization and lysosomal targeting are required for enhanced anti-tumour protective immunity in a human papillomavirus E7-expressing animal tumour model
- PMID: 16162274
- PMCID: PMC1817814
- DOI: 10.1111/j.1365-2567.2005.02219.x
Both antigen optimization and lysosomal targeting are required for enhanced anti-tumour protective immunity in a human papillomavirus E7-expressing animal tumour model
Abstract
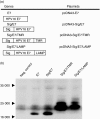
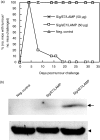
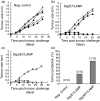
DNA immunization is a new approach for cancer immune therapy. In this study, we constructed human papillomavirus (HPV) 16 E7 expression vector cassettes and then compared the abilities of these constructs to induce antitumour protection. Lysosome-targeted E7 antigens, and to a lesser degree signal sequence-conjugated and transmembrane region sequence-conjugated E7 antigens in a DNA form, displayed tumour protection significantly higher than wild-type E7 antigens. This enhanced tumour protection was mediated by CD8+ cytotoxic T lymphocytes (CTL), as determined by in vivo T-cell depletion and in vitro interferon-gamma (IFN-gamma) production. Subsequent co-injection with interleukin-12-expressing cDNA showed insignificantly enhanced antitumour protection. However, E7 codon optimization plus lysosomal targeting resulted in a dramatic enhancement in antitumour protection both prophylactically and therapeutically through augmentation of the E7-specific CTL population, compared to either one of them alone. However, wild-type or codonoptimized E7 antigens without intracellular targeting displayed no protection against tumour challenge. Thus, these data suggest that antigen codon optimization plus lysosomal targeting strategy could be important in crafting more efficacious E7 DNA vaccines for tumour protection.
Figures

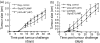
References
-
- zur Hausen H. Human papillomaviruses in the pathogenesis of anogenital cancer. Virology. 1991;184:9–13. - PubMed
-
- Werness BA, Levine AJ, Howley PM. Association of HPV type 16 and 18, E6 protein with p53. Science. 1990;248:76–9. - PubMed
-
- Dyson N, Howley PM, Munger K, Harlow E. The human papillomavirus-16, E7 oncoprotein is able to bind the retinoblastoma gene product. Science. 1989;243:934–7. - PubMed
-
- de Gruijl TD, Bontkes HJ, Stukart MJ, et al. T cell proliferative responses against human papillomavirus type 16, E7 oncoprotein are most prominent in cervical intraepithelial neoplasia patients with a persistent viral infection. J Gen Virol. 1996;77:2183–91. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

