Tyrosine kinase activity and remodelling of the actin cytoskeleton are co-temporally required for degranulation by cytotoxic T lymphocytes
- PMID: 16162276
- PMCID: PMC1817816
- DOI: 10.1111/j.1365-2567.2005.02222.x
Tyrosine kinase activity and remodelling of the actin cytoskeleton are co-temporally required for degranulation by cytotoxic T lymphocytes
Abstract
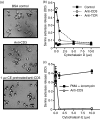
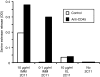
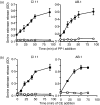
In this study, we examined the contribution of the actin cytoskeleton to T-cell receptor (TCR)-initiated signalling in cytotoxic T lymphocytes (CTLs). We demonstrate that cytoskeletal remodelling is required for sustaining TCR-stimulated signals that lead to degranulation by CTLs. Disruption of the actin cytoskeleton in CTLs already undergoing signalling responses results in an almost immediate loss of essentially all protein tyrosine phosphorylation. This signal reversal is not restricted to tyrosine phosphorylation, as disruption of the actin cytoskeleton also reverses the phosphorylation of the more downstream serine/threonine kinase extracellular signal regulated kinase (Erk). An intact cytoskeleton and cell spreading are not sufficient for maintaining signals, as stabilization of actin filaments, at a point when peak tyrosine phosphorylation is occurring, also leads to the rapid loss of protein tyrosine phosphorylation. Disruption of tyrosine kinase activity after TCR signals are maximally induced causes the immediate reversal of tyrosine phosphorylation as well as cytoskeletal disruption, as indicated by loss of cell spreading, adhesion and CTL degranulation. Taken together, our results indicate that actin remodelling occurs co-temporally with ongoing tyrosine kinase activity, leading to CTL degranulation. We hypothesize that continuous actin remodelling is important for sustaining productive signals, even after downstream signalling molecules such as Erk have been activated, and that the actin cytoskeleton is not solely required for initiating and maintaining the T cell in contact with its stimulus.
Figures




References
-
- Miletic AV, Swat M, Fujikawa K, Swat W. Cytoskeletal remodeling in lymphocyte activation. Curr Opin Immunol. 2003;15:261–8. - PubMed
-
- Fuller CL, Braciale VL, Samelson LE. All roads lead to actin: the intimate relationship between TCR signaling and the cytoskeleton. Immunol Rev. 2003;191:220–36. - PubMed
-
- Kupfer A, Singer SJ. Cell biology of cytotoxic and helper T cell functions: immunofluorescence microscopic studies of single cells and cell couples. Ann Rev Immunol. 1989;7:309–37. - PubMed
-
- Parsey MV, Lewis GK. Actin polymerization and pseudopod reorganization accompany anti-CD3-induced growth arrest in Jurkat T cells. J Immunol. 1993;151:1881–93. - PubMed
-
- Bunnell SC, Kapoor V, Trible RP, Zhang W, Samelson LE. Dynamic actin polymerization drives T cell receptor-induced spreading: a role for the signal transduction adaptor LAT. Immunity. 2001;14:315–29. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

