Dichlorination of a pyrrolyl-S-carrier protein by FADH2-dependent halogenase PltA during pyoluteorin biosynthesis
- PMID: 16162666
- PMCID: PMC1236592
- DOI: 10.1073/pnas.0506964102
Dichlorination of a pyrrolyl-S-carrier protein by FADH2-dependent halogenase PltA during pyoluteorin biosynthesis
Abstract
The antifungal natural product pyoluteorin contains a 4,5-dichloropyrrole moiety. The timing of dichlorination in the heteroaromatic ring is now shown to occur after proline is tethered by thioester linkage to the carrier protein PltL and enzymatically desaturated to the pyrrolyl-S-PltL. Surprisingly, the FADH2-dependent halogenase PltA catalyzes chlorination at both positions of the ring, generating the 5-chloropyrrolyl-S-PltL intermediate and then the 4,5-dichloropyrrolyl-S-PltL product. PltA activity strictly depends on a heterologous flavin reductase that uses NAD(P)H to produce FADH2. Electrospray ionization-Fourier transform MS detected five covalent intermediates attached to the 11-kDa carrier protein PltL. Tandem MS localized the site of covalent modification on the carrier protein scaffold. HPLC analysis of the hydrolyzed products was consistent with the regiospecific chlorination at position 5 and then position 4 of the heteroaromatic ring. A mechanism for dichlorination is proposed involving formation of a FAD-4a-OCl intermediate for capture by the electron-rich C4 and C5 of the heteroaromatic pyrrole moiety.
Figures
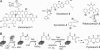



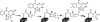
References
-
- Gribble, G. W. (2004) J. Chem. Ed. 81, 1441–1449.
-
- Liu, W., Christenson, S. D., Standage, S. & Shen, B. (2002) Science 297, 1170–1173. - PubMed
-
- Edwards, D. J., Marquez, B. L., Nogle, L. M., McPhail, K., Goeger, D. E., Roberts, M. A. & Gerwick, W. H. (2004) Chem. Biol. 11, 817–833. - PubMed
-
- Eustaquio, A. S., Gust, B., Luft, T., Li, S. M., Chater, K. F. & Heide, L. (2003) Chem. Biol. 10, 279–288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

