Repair of the injured adult hippocampus through graft-mediated modulation of the plasticity of the dentate gyrus in a rat model of temporal lobe epilepsy
- PMID: 16162921
- PMCID: PMC6725675
- DOI: 10.1523/JNEUROSCI.1538-05.2005
Repair of the injured adult hippocampus through graft-mediated modulation of the plasticity of the dentate gyrus in a rat model of temporal lobe epilepsy
Abstract
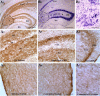
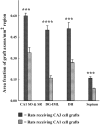
Intracerebroventricular kainate administration in rat, a model of temporal lobe epilepsy (TLE), causes degeneration of the hippocampal CA3 pyramidal and dentate hilar neurons. This leads to a robust but aberrant sprouting of the granule cell axons (mossy fibers) into the dentate supragranular layer and the CA3 stratum oriens. Because this plasticity is linked to an increased seizure susceptibility in TLE, strategies that restrain the aberrant mossy fiber sprouting (MFS) are perceived to be important for preventing the TLE development after the hippocampal injury. We ascertained the efficacy of fetal hippocampal CA3 or CA1 cell grafting into the kainate-lesioned CA3 region of the adult rat hippocampus at early post-kainic acid injury for providing a lasting inhibition of the aberrant MFS. Analyses at 12 months after grafting revealed that host mossy fibers project vigorously into CA3 cell grafts but avoid CA1 cell grafts. Consequently, in animals receiving CA3 cell grafts, the extent of aberrant MFS was minimal, in comparison with the robust MFS observed in both "lesion-only" animals and animals receiving CA1 cell grafts. Analyses of the graft axon growth revealed strong graft efferent projections into the dentate supragranular layer with CA3 cell grafting but not with CA1 cell grafting. Thus, the formation of reciprocal circuitry between the dentate granule cells and the grafted CA3 pyramidal neurons is likely the basis of inhibition of the aberrant MFS by CA3 cell grafts. The results also underscore that grafting of cells capable of differentiating into CA3 pyramidal neurons is highly efficacious for a lasting inhibition of the abnormal mossy fiber circuitry development in the injured hippocampus.
Figures




Comment in
-
Not every graft has what it takes to attract a mossy fiber.J Neurosci. 2005 Nov 9;25(45):10337-8. doi: 10.1523/JNEUROSCI.4114-05.2005. J Neurosci. 2005. PMID: 16280567 Free PMC article. No abstract available.
References
-
- Amaral DA, Witter M (1989) The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience 31: 571–591. - PubMed
-
- Babb TL, Kupfer WR, Pretorius JK, Crandall PH, Levesque MF (1991) Synaptic reorganization by mossy fibers in human epileptic fascia dentata. Neuroscience 42: 351–363. - PubMed
-
- Binder DK, Croll SD, Gall CM, Scharfman HE (2001) BDNF and epilepsy: too much of a good thing? Trends Neurosci 24: 47–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
