Brainstem and forebrain contributions to the generation of learned motor behaviors for song
- PMID: 16162936
- PMCID: PMC6725679
- DOI: 10.1523/JNEUROSCI.1668-05.2005
Brainstem and forebrain contributions to the generation of learned motor behaviors for song
Erratum in
- J Neurosci. 2005 Sep 28;25(39):table of contents
Abstract
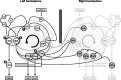
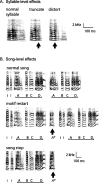
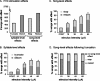
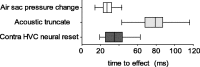
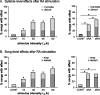
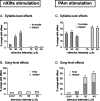
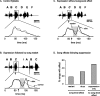
Brainstem nuclei have well established roles in generating nonlearned rhythmic behaviors or as output pathways for more complex, forebrain-generated behaviors. However, the role of the brainstem in providing information to the forebrain that is used to initiate or assist in the control of complex behaviors is poorly understood. In this study, we used electrical microstimulation in select nuclei of the avian song system combined with recordings of acoustic and respiratory output to examine how forebrain and brainstem nuclei interact in the generation of learned vocal motor sequences. We found that brief stimulation in the forebrain nuclei HVC (used as a proper name) and RA (robust nucleus of the arcopallium) caused a short-latency truncation of ongoing song syllables, which ultimately led to a cessation of the ongoing motor sequence. Stimulation within the brainstem inspiratory-related nucleus paraambigualis, which receives input from RA and projects back to HVC via the thalamus, caused syllable truncations and interruptions similar to those observed in HVC and RA. In contrast, stimulation in the tracheosyringal portion of the hypoglossal nucleus, which innervates the syrinx (the avian vocal organ) but possesses no known projections back into the song system, did not cause any significant changes in the song motor pattern. These findings suggest that perturbation of premotor activity in any nucleus within the recurrent song system motor network will disrupt the ongoing song motor sequence. Given the anatomical organization of this network, our results are consistent with a model in which the brainstem respiratory nuclei form an integral part of the song motor programming network by providing timing signals to song control nuclei in the forebrain.
Figures


References
-
- Ashmore RC, Wild JM, Schmidt MF (2004) The role of brainstem respiratory-vocal nuclei in the generation of vocal-motor sequences. Soc Neurosci Abstr 30: 786.16.
-
- Cardin JA, Raksin JN, Schmidt MF (2005) Sensorimotor nucleus NIf is necessary for auditory processing but not vocal motor output in the avian song system. J Neurophysiol 93: 2157–2166. - PubMed
-
- Cohen AH, Guan L, Harris J, Jung R, Kiemel T (1996) Interaction between the caudal brainstem and the lamprey central pattern generator for locomotion. Neuroscience 74: 1161–1173. - PubMed
-
- Coleman MJ, Vu ET (2005) Recovery of impaired songs following unilateral but not bilateral lesions of nucleus uvaeformis of adult zebra finches. J Neurobiol 63: 70–89. - PubMed
-
- Cynx J (1990) Experimental determination of a unit of song production in the zebra finch (Taeniopygia guttata). J Comp Psychol 104: 3–10. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
