Loss of Mtmr2 phosphatase in Schwann cells but not in motor neurons causes Charcot-Marie-Tooth type 4B1 neuropathy with myelin outfoldings
- PMID: 16162938
- PMCID: PMC6725661
- DOI: 10.1523/JNEUROSCI.2493-05.2005
Loss of Mtmr2 phosphatase in Schwann cells but not in motor neurons causes Charcot-Marie-Tooth type 4B1 neuropathy with myelin outfoldings
Abstract
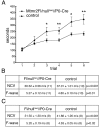
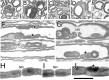
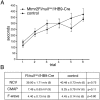
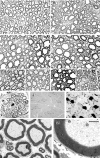
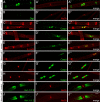
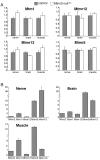
Mutations in MTMR2, the myotubularin-related 2 gene, cause autosomal recessive Charcot-Marie-Tooth type 4B1 (CMT4B1). This disorder is characterized by childhood onset of weakness and sensory loss, severely decreased nerve conduction velocity, demyelination in the nerve with myelin outfoldings, and severe functional impairment of affected patients, mainly resulting from loss of myelinated fibers in the nerve. We recently generated Mtmr2-null(neo) mice, which show a dysmyelinating neuropathy with myelin outfoldings, thus reproducing human CMT4B1. Mtmr2 is detected in both Schwann cells and neurons, in which it interacts with discs large 1/synapse-associated protein 97 and neurofilament light chain, respectively. Here, we specifically ablated Mtmr2 in either Schwann cells or motor neurons. Disruption of Mtmr2 in Schwann cells produced a dysmyelinating phenotype very similar to that of the Mtmr2-null(neo) mouse. Disruption of Mtmr2 in motor neurons does not provoke myelin outfoldings nor axonal defects. We propose that loss of Mtmr2 in Schwann cells, but not in motor neurons, is both sufficient and necessary to cause CMT4B1 neuropathy. Thus, therapeutical approaches might be designed in the future to specifically deliver the Mtmr2 phospholipid phosphatase to Schwann cells in affected nerves.
Figures



References
-
- Antonellis A, Ellsworth RE, Sambuughin N, Puls I, Abel A, Lee-Lin SQ, Jordanova A, Kremensky I, Christodoulou K, Middleton LT, Sivakumar K, Ionasescu V, Funalot B, Vance JM, Goldfarb LG, Fischbeck KH, Green ED (2003) Glycyl tRNA synthetase mutations in Charcot-Marie-Tooth disease type 2D and distal spinal muscular atrophy type V. Am J Hum Genet 72: 1293–1299. - PMC - PubMed
-
- Arber S, Han B, Mendelsohn M, Smith M, Jessell TM, Sockanathan S (1999) Requirement for the homeobox gene HB9 in the consolidation of motor neuron identity. Neuron 23: 659–674. - PubMed
-
- Azzedine H, Bolino A, Taieb T, Birouk N, Di Duca M, Bouhouche A, Benamou S, Mrabet A, Hammadouche T, Chkili T, Gouider R, Ravazzolo R, Brice A, Laporte J, LeGuern E (2003) Mutations in MTMR13, a new pseudophosphatase homologue of MTMR2 and Sbf1, in two families with an autosomal recessive demyelinating form of Charcot-Marie-Tooth disease associated with early-onset glaucoma. Am J Hum Genet 72: 1141–1153. - PMC - PubMed
-
- Bolino A, Muglia M, Conforti FL, LeGuern E, Salih MA, Georgiou DM, Christodoulou K, Hausmanowa-Petrusewicz I, Mandich P, Schenone A, Gambardella A, Bono F, Quattrone A, Devoto M, Monaco AP (2000) Charcot-Marie-Tooth type 4B is caused by mutations in the gene encoding myotubularin-related protein-2. Nat Genet 25: 17–19. - PubMed
-
- Bolino A, Marigo V, Ferrera F, Loader J, Romio L, Leoni A, Di Duca M, Cinti R, Cecchi C, Feltri ML, Wrabetz L, Ravazzolo R, Monaco AP (2002) Molecular characterization and expression analysis of Mtmr2, mouse homologue of MTMR2, the Myotubularin-related 2 gene, mutated in CMT4B. Gene 283: 17–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
