ATM and Chk2-dependent phosphorylation of MDMX contribute to p53 activation after DNA damage
- PMID: 16163388
- PMCID: PMC1276172
- DOI: 10.1038/sj.emboj.7600812
ATM and Chk2-dependent phosphorylation of MDMX contribute to p53 activation after DNA damage
Abstract
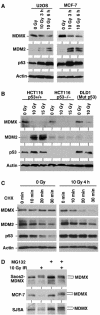
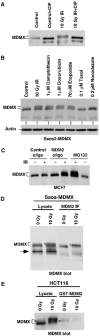
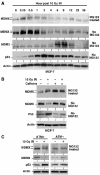
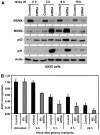
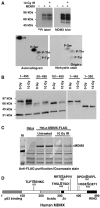
The p53 tumor suppressor is activated after DNA damage to maintain genomic stability and prevent transformation. Rapid activation of p53 by ionizing radiation is dependent on signaling by the ATM kinase. MDM2 and MDMX are important p53 regulators and logical targets for stress signals. We found that DNA damage induces ATM-dependent phosphorylation and degradation of MDMX. Phosphorylated MDMX is selectively bound and degraded by MDM2 preceding p53 accumulation and activation. Reduction of MDMX level by RNAi enhances p53 response to DNA damage. Loss of ATM prevents MDMX degradation and p53 stabilization after DNA damage. Phosphorylation of MDMX on S342, S367, and S403 were detected by mass spectrometric analysis, with the first two sites confirmed by phosphopeptide-specific antibodies. Mutation of MDMX on S342, S367, and S403 each confers partial resistance to MDM2-mediated ubiquitination and degradation. Phosphorylation of S342 and S367 in vivo require the Chk2 kinase. Chk2 also stimulates MDMX ubiquitination and degradation by MDM2. Therefore, the E3 ligase activity of MDM2 is redirected to MDMX after DNA damage and contributes to p53 activation.
Figures


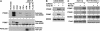

References
-
- Ahn J, Urist M, Prives C (2003) Questioning the role of checkpoint kinase 2 in the p53 DNA damage response. J Biol Chem 278: 20480–20489 - PubMed
-
- Bakkenist CJ, Kastan MB (2003) DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421: 499–506 - PubMed
-
- Banin S, Moyal L, Shieh S-Y, Taya Y, Anderson CW, Chessa L, Smorodinsky NI, Prives C, Reiss Y, Shiloh Y, Ziv Y (1998) Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science 281: 1674–1677 - PubMed
-
- Bartek J, Lukas J. (2003) Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell 3: 421–449 - PubMed
-
- Chao C, Hergenhahn M, Kaeser MD, Wu Z, Saito S, Iggo R, Hollstein M, Appella E, Xu Y (2003) Cell type- and promoter-specific roles of Ser18 phosphorylation in regulating p53 responses. J Biol Chem 278: 41028–41033 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

