Far-red fluorescent proteins evolved from a blue chromoprotein from Actinia equina
- PMID: 16164420
- PMCID: PMC1316306
- DOI: 10.1042/BJ20051314
Far-red fluorescent proteins evolved from a blue chromoprotein from Actinia equina
Abstract
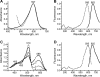
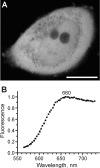
Proteins of the GFP (green fluorescent protein) family demonstrate a great spectral and phylogenetic diversity. However, there is still an intense demand for red-shifted GFP-like proteins in both basic and applied science. To obtain GFP-like chromoproteins with red-shifted absorption, we performed a broad search in blue-coloured Anthozoa species. We revealed specimens of Actinia equina (beadlet anemone) exhibiting a bright blue circle band at the edge of the basal disc. A novel blue chromoprotein, aeCP597, with an absorption maximum at 597 nm determining the coloration of the anemone basal disk was cloned. AeCP597 carries a chromophore chemically identical with that of the well-studied DsRed (red fluorescent protein from Discosoma sp.). Thus a strong 42-nm bathochromic shift of aeCP597 absorption compared with DsRed is determined by peculiarities of chromophore environment. Site-directed and random mutagenesis of aeCP597 resulted in far-red fluorescent mutants with emission maxima at up to 663 nm. The most bright and stable mutant AQ143 possessed excitation and emission maxima at 595 and 655 nm respectively. Thus aeCP597 and its fluorescent mutants set a new record of red-shifted absorption and emission maxima among GFP-like proteins.
Figures


References
-
- Lippincott-Schwartz J., Patterson G. H. Development and use of fluorescent protein markers in living cells. Science. 2003;300:87–91. - PubMed
-
- Verkhusha V. V., Lukyanov K. A. The molecular properties and applications of Anthozoa fluorescent proteins and chromoproteins. Nat. Biotechnol. 2004;22:289–296. - PubMed
-
- Shagin D. A., Barsova E. V., Yanushevich Y. G., Fradkov A. F., Lukyanov K. A., Labas Y. A., Semenova T. N., Ugalde J. A., Meyers A., Nunez J. M., et al. GFP-like proteins as ubiquitous metazoan superfamily: evolution of functional features and structural complexity. Mol. Biol. Evol. 2004;21:841–850. - PubMed
-
- Matz M. V., Fradkov A. F., Labas Y. A., Savitsky A. P., Zaraisky A. G., Markelov M. L., Lukyanov S. A. Fluorescent proteins from nonbioluminescent Anthozoa species. Nat. Biotechnol. 1999;17:969–973. - PubMed
-
- Lukyanov K. A., Fradkov A. F., Gurskaya N. G., Matz M. V., Labas Y. A., Savitsky A. P., Markelov M. L., Zaraisky A. G., Zhao X., Fang Y., et al. Natural animal coloration can be determined by a non-fluorescent green fluorescent protein homolog. J. Biol. Chem. 2000;275:25879–25882. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

