Deciphering the late steps in the biosynthesis of the anti-tumour indolocarbazole staurosporine: sugar donor substrate flexibility of the StaG glycosyltransferase
- PMID: 16164546
- PMCID: PMC2881644
- DOI: 10.1111/j.1365-2958.2005.04777.x
Deciphering the late steps in the biosynthesis of the anti-tumour indolocarbazole staurosporine: sugar donor substrate flexibility of the StaG glycosyltransferase
Abstract
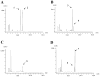
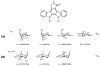
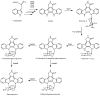
The indolocarbazole staurosporine is a potent inhibitor of a variety of protein kinases. It contains a sugar moiety attached through C-N linkages to both indole nitrogen atoms of the indolocarbazole core. Staurosporine biosynthesis was reconstituted in vivo in a heterologous host Streptomyces albus by using two different plasmids: the 'aglycone vector' expressing a set of genes involved in indolocarbazole biosynthesis together with staG (encoding a glycosyltransferase) and/or staN (coding for a P450 oxygenase), and the 'sugar vector' expressing a set of genes responsible for the biosynthesis of the sugar moiety. Attachment of the sugar to the two indole nitrogens of the indolocarbazole core was dependent on the combined action of StaG and StaN. When StaN was absent, the sugar was attached only to one of the nitrogen atoms, through an N-glycosidic linkage, as in the indolocarbazole rebeccamycin. The StaG glycosyltransferase showed flexibility with respect to the sugar donor. When the 'sugar vector' was substituted by constructs directing the biosynthesis of l-rhamnose, L-digitoxose, L-olivose and D-olivose, respectively, StaG and StaN were able to transfer and attach all of these sugars to the indolocarbazole aglycone.
Figures



Comment in
-
It works: combinatorial biosynthesis for generating novel glycosylated compounds.Mol Microbiol. 2005 Oct;58(1):3-5. doi: 10.1111/j.1365-2958.2005.04815.x. Mol Microbiol. 2005. PMID: 16164544
References
-
- Akinaga S, Sugiyama K, Akiyama T. UCN-01 (7-hydroxystaurosporine) and other indolocarbazole compounds: a new generation of anticancer agents for the new century? Anticancer Drug Des. 2000;15:43–52. - PubMed
-
- Albermann C, Soriano A, Jiang J, Vollmer H, Biggins JB, Barton WA, et al. Substrate specificity of NovM: implications for novobiocin biosynthesis and glycorandomization. Org Lett. 2003;5:933–936. - PubMed
-
- Bailly C, Qu X, Graves DE, Prudhomme M, Chaires JB. Calories from carbohydrates: energetic contribution of the carbohydrate moiety of rebeccamycin to DNA binding and the effect of its orientation on topoisomerase I inhibition. Chem Biol. 1999;6:277–286. - PubMed
-
- Blanco G, Patallo EP, Braña AF, Trefzer A, Bechthold A, Rohr J, et al. Identification of a sugar flexible glycosyltransferase from Streptomyces olivaceus, the producer of the antitumor polyketide elloramycin. Chem Biol. 2001;8:253–263. - PubMed
-
- Cai Y, Fredenhagen A, Hug P, Peter HH. A nitro analogue of staurosporine and other minor metabolites produced by a Streptomyces longisporoflavus strain. J Antibiot. 1995;48:143–148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

