Comparative analysis of the kinomes of three pathogenic trypanosomatids: Leishmania major, Trypanosoma brucei and Trypanosoma cruzi
- PMID: 16164760
- PMCID: PMC1266030
- DOI: 10.1186/1471-2164-6-127
Comparative analysis of the kinomes of three pathogenic trypanosomatids: Leishmania major, Trypanosoma brucei and Trypanosoma cruzi
Abstract
Background: The trypanosomatids Leishmania major, Trypanosoma brucei and Trypanosoma cruzi cause some of the most debilitating diseases of humankind: cutaneous leishmaniasis, African sleeping sickness, and Chagas disease. These protozoa possess complex life cycles that involve development in mammalian and insect hosts, and a tightly coordinated cell cycle ensures propagation of the highly polarized cells. However, the ways in which the parasites respond to their environment and coordinate intracellular processes are poorly understood. As a part of an effort to understand parasite signaling functions, we report the results of a genome-wide analysis of protein kinases (PKs) of these three trypanosomatids.
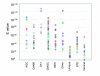
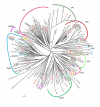
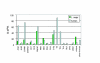
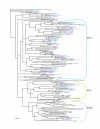
Results: Bioinformatic searches of the trypanosomatid genomes for eukaryotic PKs (ePKs) and atypical PKs (aPKs) revealed a total of 176 PKs in T. brucei, 190 in T. cruzi and 199 in L. major, most of which are orthologous across the three species. This is approximately 30% of the number in the human host and double that of the malaria parasite, Plasmodium falciparum. The representation of various groups of ePKs differs significantly as compared to humans: trypanosomatids lack receptor-linked tyrosine and tyrosine kinase-like kinases, although they do possess dual-specificity kinases. A relative expansion of the CMGC, STE and NEK groups has occurred. A large number of unique ePKs show no strong affinity to any known group. The trypanosomatids possess few ePKs with predicted transmembrane domains, suggesting that receptor ePKs are rare. Accessory Pfam domains, which are frequently present in human ePKs, are uncommon in trypanosomatid ePKs.
Conclusion: Trypanosomatids possess a large set of PKs, comprising approximately 2% of each genome, suggesting a key role for phosphorylation in parasite biology. Whilst it was possible to place most of the trypanosomatid ePKs into the seven established groups using bioinformatic analyses, it has not been possible to ascribe function based solely on sequence similarity. Hence the connection of stimuli to protein phosphorylation networks remains enigmatic. The presence of numerous PKs with significant sequence similarity to known drug targets, as well as a large number of unusual kinases that might represent novel targets, strongly argue for functional analysis of these molecules.
Figures




References
-
- TDR Homepage. 2005. http://www.who.int/tdr
-
- Murray HW. Treatment of visceral leishmaniasis in 2004. Am J Trop Med Hyg. 2004;71:787–794. - PubMed
-
- El-Sayed NMA, Myler PJ, Bartholomeu D, Nilsson D, Aggarwal G, Tran AN, Ghedin E, Worthey EA, Delcher A, Blandin G, Westenberger S, Haas B, Caler E, Cerqueira G, Arner E, Aslund L, Bontempi E, Branche C, Bringaud F, Campbell D, Carrington M, Crabtree JS, Darban H, Edwards K, Englund P, Feldblyum T, Ferella M, Frasch C, Kindlund E, Klingbeil MM, Kluge S, Koo HL, Lacerda D, McCulloch R, McKenna A, Mizuno Y, Mottram J, Ochaya S, Pai G, Parsons M, Pettersson U, Pop M, Luis Ramirez J, Salzberg S, Tammi M, Tarleton RL, Teixeira SM, Van Aken S, Wortman J, Stuart KD, Andersson B, Anapuma A, Attipoe P, Burton P, Cadag E, Franco da Silva J, de Jong P, Fazelinia G, Gull K, Horn D, Hou L, Huang Y, Levin MJ, Lorenzi H, Louie T, Machado CR, Nelson S, Osoegawa K, Pentony M, Rinta J, Robertson L, Sanchez DO, Seyler A, Sharma R, Shetty J, Simpson AJ, Sisk E, Vogt C, Ward P, Wickstead B, White O, Fraser CM, Stuart KD, Andersson B. The genome sequence of Trypanosoma cruzi, etiological agent of Chagas' disease. Science. 2005;309:409–415. doi: 10.1126/science.1112631. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

