Spike coding during osmotic stimulation of the rat supraoptic nucleus
- PMID: 16166154
- PMCID: PMC1464216
- DOI: 10.1113/jphysiol.2005.097014
Spike coding during osmotic stimulation of the rat supraoptic nucleus
Abstract
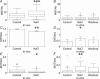
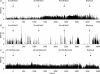
Novel measures of coding based on interspike intervals were used to characterize the responses of supraoptic cells to osmotic stimulation. Infusion of hypertonic NaCl in vivo increased the firing rate of continuous (putative oxytocin) cells (Wilcoxon z= 3.84, P= 0.001) and phasic (putative vasopressin) cells (z= 2.14, P= 0.032). The irregularity of activity, quantified by the log interval entropy, was decreased for continuous (Student's t= 3.06, P= 0.003) but not phasic cells (t= 1.34, P= 0.181). For continuous cells, the increase in frequency and decrease in entropy was significantly greater (t= 2.61, P= 0.036 and t= 3.06, P= 0.007, respectively) than for phasic cells. Spike patterning, quantified using the mutual information between intervals, was decreased for phasic (z=-2.64, P= 0.008) but not continuous cells (z=-1.14, P= 0.256). Although continuous cells showed similar osmotic responses to mannitol infusion, phasic cells showed differences: spike frequency decreased (z=-3.70, P < 0.001) and entropy increased (t=-3.41, P < 0.001). Considering both cell types together, osmotic stimulation in vitro using 40 mm NaCl had little effect on firing rate (z=-0.319, P= 0.750), but increased both entropy (t= 2.75, P= 0.010) and mutual information (z=-2.73, P= 0.006) in contrast to the decreases (t= 2.92, P= 0.004 and z=-2.40, P= 0.017) seen in vivo. Responses to less severe osmotic stimulation with NaCl or mannitol were not significant. Potassium-induced depolarization in vitro increased firing rate (r= 0.195, P= 0.034), but the correlation with decreased entropy was not significant (r=-0.097, P= 0.412). Intracellular recordings showed a small depolarization and decrease in input resistance during osmotic stimulation with NaCl or mannitol, and membrane depolarization following addition of potassium. Differences in responses of oxytocin and vasopressin cells in vivo, suggest differences in the balance between the synaptic and membrane properties involved in coding their osmotic responses. The osmotic responses in vivo constrasted with those seen in vitro, which suggests that, in vivo, they depend on extrinsic circuitry. Differences in responses to osmolality and direct depolarization in vitro indicate that the mechanism of osmoresponsiveness within a physiological range is unlikely to be fully explained by depolarization.
Figures









Similar articles
-
Daily rhythms of spike coding in the rat supraoptic nucleus.J Neuroendocrinol. 2009 Nov;21(11):935-45. doi: 10.1111/j.1365-2826.2009.01918.x. J Neuroendocrinol. 2009. PMID: 19863704
-
Assessment of spike activity in the supraoptic nucleus.J Neuroendocrinol. 2004 Apr;16(4):390-7. doi: 10.1111/j.0953-8194.2004.01166.x. J Neuroendocrinol. 2004. PMID: 15089980 Review.
-
Phasic spike patterning in rat supraoptic neurones in vivo and in vitro.J Physiol. 2004 Jul 1;558(Pt 1):161-80. doi: 10.1113/jphysiol.2004.063982. Epub 2004 May 14. J Physiol. 2004. PMID: 15146047 Free PMC article.
-
Oxytocin release following osmotic activation of oxytocin neurones in the paraventricular and supraoptic nuclei.J Physiol. 1978 May;278:69-78. doi: 10.1113/jphysiol.1978.sp012293. J Physiol. 1978. PMID: 209173 Free PMC article.
-
Circadian modulation of osmoregulated firing in rat supraoptic nucleus neurones.J Neuroendocrinol. 2012 Apr;24(4):577-86. doi: 10.1111/j.1365-2826.2012.02298.x. J Neuroendocrinol. 2012. PMID: 22330181 Review.
Cited by
-
Oxytocin Neurones: Intrinsic Mechanisms Governing the Regularity of Spiking Activity.J Neuroendocrinol. 2016 Apr;28(4):n/a. doi: 10.1111/jne.12358. J Neuroendocrinol. 2016. PMID: 26715365 Free PMC article.
-
Age affects spontaneous activity and depolarizing afterpotentials in isolated gonadotropin-releasing hormone neurons.Endocrinology. 2008 Oct;149(10):4938-47. doi: 10.1210/en.2008-0308. Epub 2008 Jun 26. Endocrinology. 2008. PMID: 18583421 Free PMC article.
-
Dehydration-induced modulation of kappa-opioid inhibition of vasopressin neurone activity.J Physiol. 2009 Dec 1;587(Pt 23):5679-89. doi: 10.1113/jphysiol.2009.180232. Epub 2009 Oct 12. J Physiol. 2009. PMID: 19822541 Free PMC article.
-
Apamin increases post-spike excitability of supraoptic nucleus neurons in anaesthetized morphine-naïve rats and morphine-dependent rats: consequences for morphine withdrawal excitation.Exp Brain Res. 2011 Aug;212(4):517-28. doi: 10.1007/s00221-011-2759-z. Epub 2011 Jun 14. Exp Brain Res. 2011. PMID: 21671103
-
Probabilistic identification of cerebellar cortical neurones across species.PLoS One. 2013;8(3):e57669. doi: 10.1371/journal.pone.0057669. Epub 2013 Mar 4. PLoS One. 2013. PMID: 23469215 Free PMC article.
References
-
- Andrew RD, Dudek FE. Analysis of intracellularly recorded phasic bursting by mammalian neuroendocrine cells. J Neurophysiol. 1984;51:552–566. - PubMed
-
- Bhumbra GS, Dyball REJ. Spike coding from the perspective of a neurone. Cogn Processing. 2005;6:157–176. - PubMed
-
- Bhumbra GS, Inyushkin AN, Dyball REJ. Assessment of spike activity in the supraoptic nucleus. J Neuroendocrinol. 2004;16:390–397. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

