Cochlear function in mice with only one copy of the prestin gene
- PMID: 16166160
- PMCID: PMC1464211
- DOI: 10.1113/jphysiol.2005.093518
Cochlear function in mice with only one copy of the prestin gene
Abstract
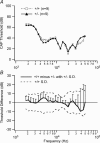
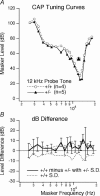
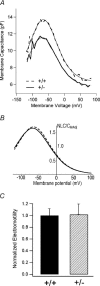
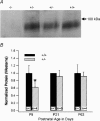
Targeted deletion of the prestin gene reduces cochlear sensitivity and eliminates both frequency selectivity and outer hair cell (OHC) somatic electromotility. In addition, it has been reported by Liberman and colleagues that F2 generation heterozygotes exhibit a 6 dB reduction in sensitivity, as well as a decrease in protein and electromotility. Considering that the active process is non-linear, a halving of somatic electromotility would be expected to produce a much larger change in sensitivity. We therefore re-evaluated comparisons between heterozygotes and wildtype mice using both in vivo and in vitro electrophysiology, as well as molecular biology. Data reported here for F3-F5 generation mice indicate that compound action potential thresholds and tuning curves, as well as the cochlear microphonic, are similar in heterozygotes and wildtype controls. Measurements of non-linear capacitance in isolated OHCs demonstrate that charge density, as well as the voltage dependence and sensitivity of motor function, is indistinguishable in the two genotypes, as is somatic electromotility. In addition, both immunocytochemistry and western blot analysis in young adult mice suggest that prestin protein in heterozygotes is near normal. In contrast, prestin mRNA is always less than in wildtype mice at all ages tested. Results from F3-F5 generation mice suggest that one copy of the prestin gene is capable of compensating for the deleted copy and that heterozygous mice do not suffer peripheral hearing impairment.
Figures


References
-
- Ashmore JF. Forward and reverse transduction in the mammalian cochlea. Neurosci Res Suppl. 1990;12:S39–S50. - PubMed
-
- Brownell WE, Bader CR, Bertrand D, Ribaupierre Y. Evoked mechanical responses of isolated outer hair cells. Science. 1985;227:194–196. - PubMed
-
- Bustin SA. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and problems. J Mol Endocrinol. 2002;29:23–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

