Connections of thalamic modulatory centers to the vocal control system of the zebra finch
- PMID: 16166261
- PMCID: PMC1236583
- DOI: 10.1073/pnas.0506774102
Connections of thalamic modulatory centers to the vocal control system of the zebra finch
Abstract
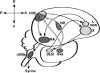
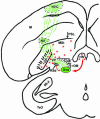
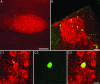
The vocal control system of zebra finches shows auditory gating in which neuronal responses to the individual bird's own song vary with behavioral states such as sleep and wakefulness. However, we know neither the source of gating signals nor the anatomical connections that could link the modulatory centers of the brain with the song system. Two of the song-control nuclei in the forebrain, the HVC (used as the proper name) and the interfacial nucleus of the nidopallium, both show auditory gating, and they receive input from the uvaeform nucleus (Uva) in the thalamus. We used a combination of anterograde and retrograde tracing methods to show that the dorsal part of the reticular formation and the medial habenula (MHb) project to the Uva. We also show by choline acetyl transferase immunohistochemistry that the MHb is cholinergic and sends cholinergic fibers to the Uva. Our findings suggest that the Uva might serve as a hub to coordinate neuromodulatory input into the song system.
Figures

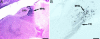

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

